The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus that causes coronavirus disease 2019 (COVID-19), is associated with high mortality and hospitalization rates. However, many patients who are infected with SARS-CoV-2 remain asymptomatic or develop mild symptoms.
 Study: Neutrophil-epithelial interactions augment infectivity and pro-inflammatory responses to SARS-CoV-2 infection. Image Credit: Kateryna Kon / Shutterstock.com
Study: Neutrophil-epithelial interactions augment infectivity and pro-inflammatory responses to SARS-CoV-2 infection. Image Credit: Kateryna Kon / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
An overview of neutrophils
Neutrophils are the first and predominant immune cells that are recruited to the respiratory tract in response to viral infection. Upon their arrival, neutrophils release various inflammatory mediators in an effort to rapidly eliminate the pathogen from the infected area.
Neutrophils are capable of recognizing infectious sites as well as act as sites of infections which, together, leads to an acute inflammatory response. An uncontrolled massive inflammatory response, which is also known as the cytokine storm, has been documented in patients with severe COVID-19.
“Despite their importance in anti-viral immunity and response to viral pathogens, neutrophils have been somewhat overlooked for their role in the pathogenesis of SARS-CoV-2 infection.”
About the study
The researchers developed a novel in vitro model to assess neutrophilic airway inflammation in SARS-CoV-2 infection. To this end, primary human airway basal epithelial cells were isolated from the lung tissue.
The current study involved the isolation of neutrophils from peripheral blood. Following their isolation, the purity of the neutrophils was confirmed by flow-activated cell sorting (FACS).
Freshly isolated neutrophils were then added to the differentiated airway epithelial cells, followed by infection with SARS-CoV-2 for a total of 4 hours.
Along with these experiments, the researchers also performed immunohistochemistry of the primary human lung tissues obtained from severe COVID-19 patients.
Study findings
The analysis of the lung pathologies of COVID-19 patients confirmed the extensive infiltration of inflammatory cells, including neutrophils. More specifically, these post-mortem samples were found to have increased neutrophil elastase (NE) activity as compared to the neutrophils that were identified in healthy lung tissues. High neutrophil invasion and epithelial shedding were also observed, which suggested that neutrophils play an important role in SARS-CoV-2 infection.
The peripheral blood samples of COVID-19 patients also showed higher concentrations of pro-inflammatory cytokines that disrupt epithelial permeability in patients with severe disease.
“Additionally, marked increases in neutrophils were also observed in bronchiolar alveolar lavage (BAL) fluids from patients admitted into the intensive care unit (ICU) compared to intermediary medical unit (IMU) patients correlating to disease severity."
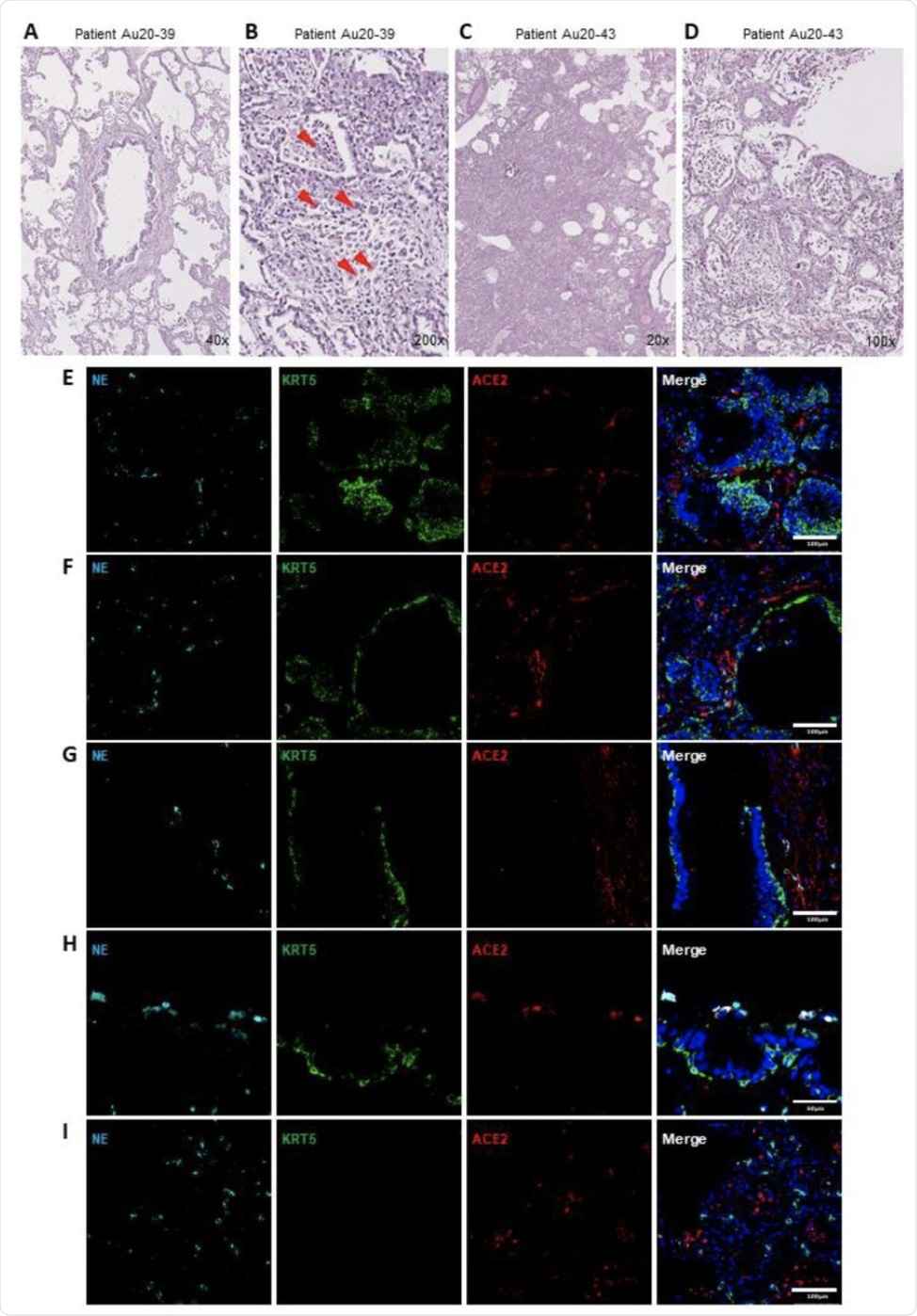 Neutrophil associated tissue pathology in post-mortem COVID19 human lung airways. (A-D) Representative images of hematoxylin and eosin (H&E) staining of postmortem COVID-19 patient tissues showing patchy organizing pneumonia centered around a major artery and an airway (A); focally expanded interstitium by a mixed cellular infiltrate including scattered giant cells (orange arrowheads) (B); diffuse alveolar damage from intense fibroinflammatory process and barotrauma induced rounded airspaces (C) and organizing diffuse alveolar damage with fibrin disposition replaced by organizing pneumonia, inflammatory cells and oedema (D). (E-H) Representative IF images of postmortem COVID-19 tissue probed for NE (cyan), KRT5 (green) and ACE2 (red). Images highlight; small airway occlusion resulting from basal cell hyperplasia with surrounding neutrophils present (E); epithelial damage with breaching neutrophils into the luminal space (F); epithelial shedding, inclusive of basal cell layer with neutrophil inclusion of mucosal surface (G); neutrophil breach into airway luminal space with high neutrophil elastase activity (H) and diffuse neutrophil invasion of alveolar spaces (I). All IF images have nuclei counterstained with DAPI (blue) and scale bars represent 100 μm. All images are representative of 3 independent regions per donor at least 2 independent donors.
Neutrophil associated tissue pathology in post-mortem COVID19 human lung airways. (A-D) Representative images of hematoxylin and eosin (H&E) staining of postmortem COVID-19 patient tissues showing patchy organizing pneumonia centered around a major artery and an airway (A); focally expanded interstitium by a mixed cellular infiltrate including scattered giant cells (orange arrowheads) (B); diffuse alveolar damage from intense fibroinflammatory process and barotrauma induced rounded airspaces (C) and organizing diffuse alveolar damage with fibrin disposition replaced by organizing pneumonia, inflammatory cells and oedema (D). (E-H) Representative IF images of postmortem COVID-19 tissue probed for NE (cyan), KRT5 (green) and ACE2 (red). Images highlight; small airway occlusion resulting from basal cell hyperplasia with surrounding neutrophils present (E); epithelial damage with breaching neutrophils into the luminal space (F); epithelial shedding, inclusive of basal cell layer with neutrophil inclusion of mucosal surface (G); neutrophil breach into airway luminal space with high neutrophil elastase activity (H) and diffuse neutrophil invasion of alveolar spaces (I). All IF images have nuclei counterstained with DAPI (blue) and scale bars represent 100 μm. All images are representative of 3 independent regions per donor at least 2 independent donors.
The findings of neutrophilia within the airways of severe COVID-19 patients led the researchers to assess how neutrophilic airway inflammation plays a role in SARS-CoV-2 infection. Prior to this determination, the researchers confirmed the expression of the angiotensin-converting enzyme 2 (ACE2) receptor and transmembrane serine protease 2 (TMPRSS2) in their novel in vitro airway epithelium model to confirm its utility in studying the infection mechanisms by SARS-CoV-2.
“Using this model, we were able to conclude that the presence of neutrophils with the airway epithelium significantly augments the proinflammatory responses to SARS-CoV-2, increases airway viral load and decreases airway epithelial barrier integrity.”
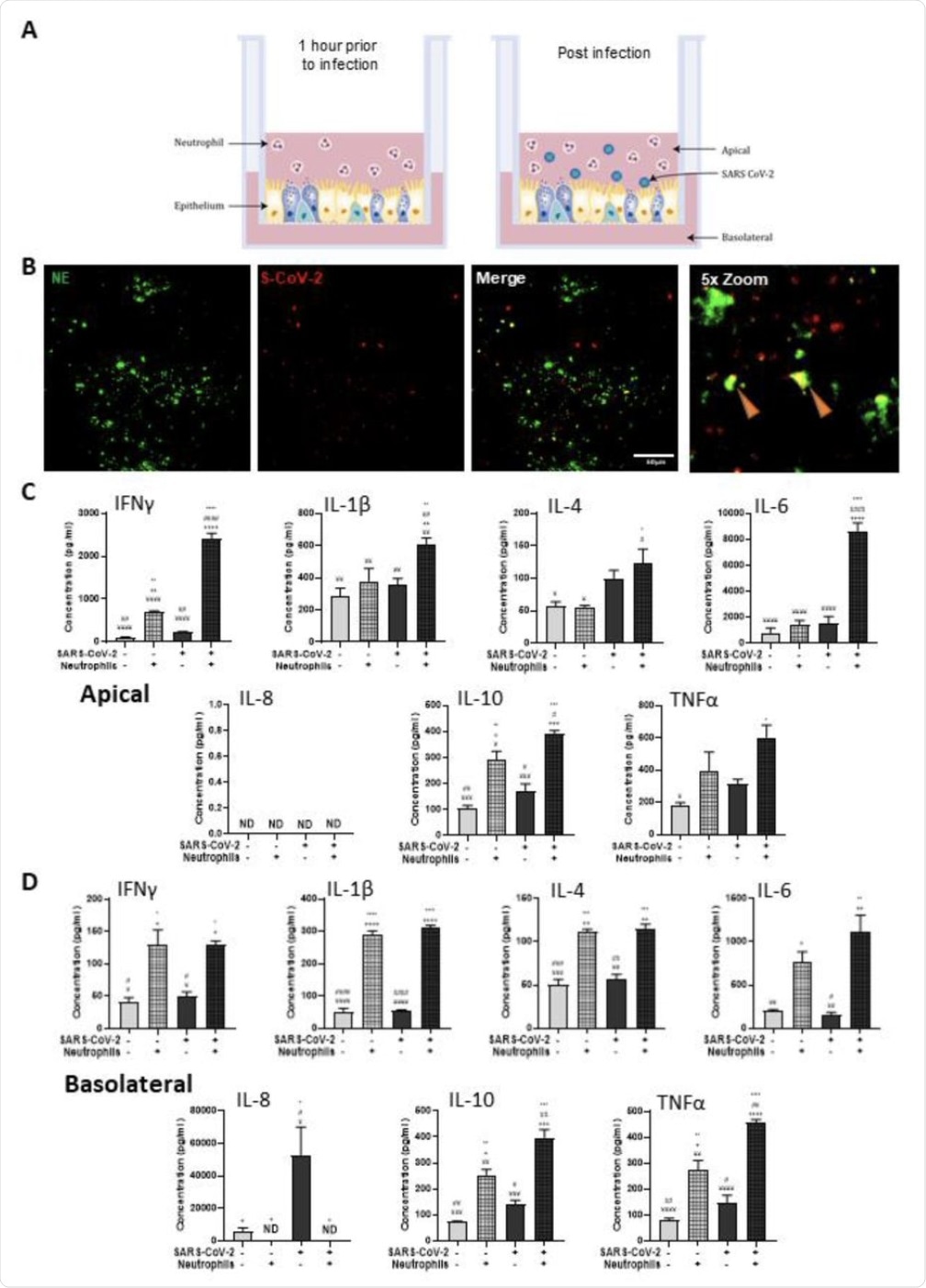 Polarized inflammatory response of neutrophils in co-culture with human airway epithelium, infected with SARS-CoV-2. (A) Schematic of the in vitro model of neutrophilic airways denoting neutrophils in co-culture with differentiated airway epithelial cells and infected with live SARS-CoV-2 virus. (B) Representative IF images of primary human airway epithelial cells differentiated at the air-liquid interface stained with NE (green) and probed by RNAScope for SARS-CoV-2 (red). Scale bars represent 100μm. Inflammatory profiles of apical (C) and basolateral (D) supernatants collected 4 hours post infection in the neutrophilic airway model. Data is expressed as mean±SEM and significance is determined by analysis of variance (ANOVA) followed by Tukey’s post hoc analysis. * compared to uninfected epithelial monoculture, # compared to uninfected epithelial and neutrophil co-culture, + compared to infected monoculture epithelial cells, ¥ significant from infected neutrophil and epithelial co-culture. *p<0.05,**p<0.01, ***p<0.001, ****,<0.0001 from n=3 experimental repeats from N=3 donors.
Polarized inflammatory response of neutrophils in co-culture with human airway epithelium, infected with SARS-CoV-2. (A) Schematic of the in vitro model of neutrophilic airways denoting neutrophils in co-culture with differentiated airway epithelial cells and infected with live SARS-CoV-2 virus. (B) Representative IF images of primary human airway epithelial cells differentiated at the air-liquid interface stained with NE (green) and probed by RNAScope for SARS-CoV-2 (red). Scale bars represent 100μm. Inflammatory profiles of apical (C) and basolateral (D) supernatants collected 4 hours post infection in the neutrophilic airway model. Data is expressed as mean±SEM and significance is determined by analysis of variance (ANOVA) followed by Tukey’s post hoc analysis. * compared to uninfected epithelial monoculture, # compared to uninfected epithelial and neutrophil co-culture, + compared to infected monoculture epithelial cells, ¥ significant from infected neutrophil and epithelial co-culture. *p<0.05,**p<0.01, ***p<0.001, ****,<0.0001 from n=3 experimental repeats from N=3 donors.
Conclusion
“In conclusion, we have developed a model to study neutrophil-epithelial interactions which more closely reflects an in vivo and more clinically relevant infection of airways in severe COVID-19 cases than do monocultures.”
The findings of this study demonstrate that the presence of neutrophils in airway epithelium leads to SARS-CoV-2 mediated pro-inflammatory cytokine release. This acute inflammatory response therefore leads to an increase in viral load and decrease in barrier integrity.
“Overall, we make important observations that reveal a key role for neutrophil-epithelial interactions in determining infectivity and outcomes in response to SARS-CoV-2 infection that highlight neutrophils as a potential target for prevention of severe COVID-19 disease”, adds the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Calvert, B. A., Quiroz, B. A., Lorenzana, Z., et al. (2021. Neutrophil-epithelial interactions augment infectivity and pro-inflammatory responses to SARS-CoV-2 infection. bioRxiv. doi:10.1101/2021.08.09.455472 . https://www.biorxiv.org/content/10.1101/2021.08.09.455472v1 .
- Peer reviewed and published scientific report.
Calvert, Ben A., Erik J. Quiroz, Zareeb Lorenzana, Ngan Doan, Seongjae Kim, Christiana N. Senger, Jeffrey J. Anders, et al. 2023. “Neutrophilic Inflammation Promotes SARS-CoV-2 Infectivity and Augments the Inflammatory Responses in Airway Epithelial Cells.” Frontiers in Immunology 14 (March). https://doi.org/10.3389/fimmu.2023.1112870. https://www.frontiersin.org/articles/10.3389/fimmu.2023.1112870/full.