Natural infection or vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) confer considerable protection against reinfection and reduce the risk of severe Coronavirus disease 2019 (COVID-19) outcomes.
However, reports on decaying neutralizing antibody (Ab) levels against SARS-CoV-2 have raised questions regarding long-term immunity. Sporadic breakthrough infections have been reported after vaccination due to lower antibody levels to SARS-CoV-2 spike protein, prompting consideration of booster doses. Therefore, urgent studies are needed to identify factors associated with protection to assist in the future deployment of vaccines.
Diana Zhong and colleagues from Johns Hopkins University have conducted a cohort study on healthcare workers (HWs) to probe SARS-CoV-2 spike Immunoglobulin G (IgG) Ab levels, comparing their decay in vaccinated individuals with and without prior SARS-CoV-2 infection.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer view.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Cohort of healthcare workers
The HWs included in the study had either a serum sample collected post-SARS-CoV-2 infection (defined as positive SARS-CoV-2 PCR) (n=98), and/or a serum sample collected ≥ 14 days after the second dose of an mRNA SARS-CoV-2 vaccine (n=1,960).
Enzyme-linked immunosorbent assay (ELISA) was used to quantify Abs in the serum and linear regression models, adjusting for vaccine type, age, and sex, were used to compare post-vaccination Ab levels.
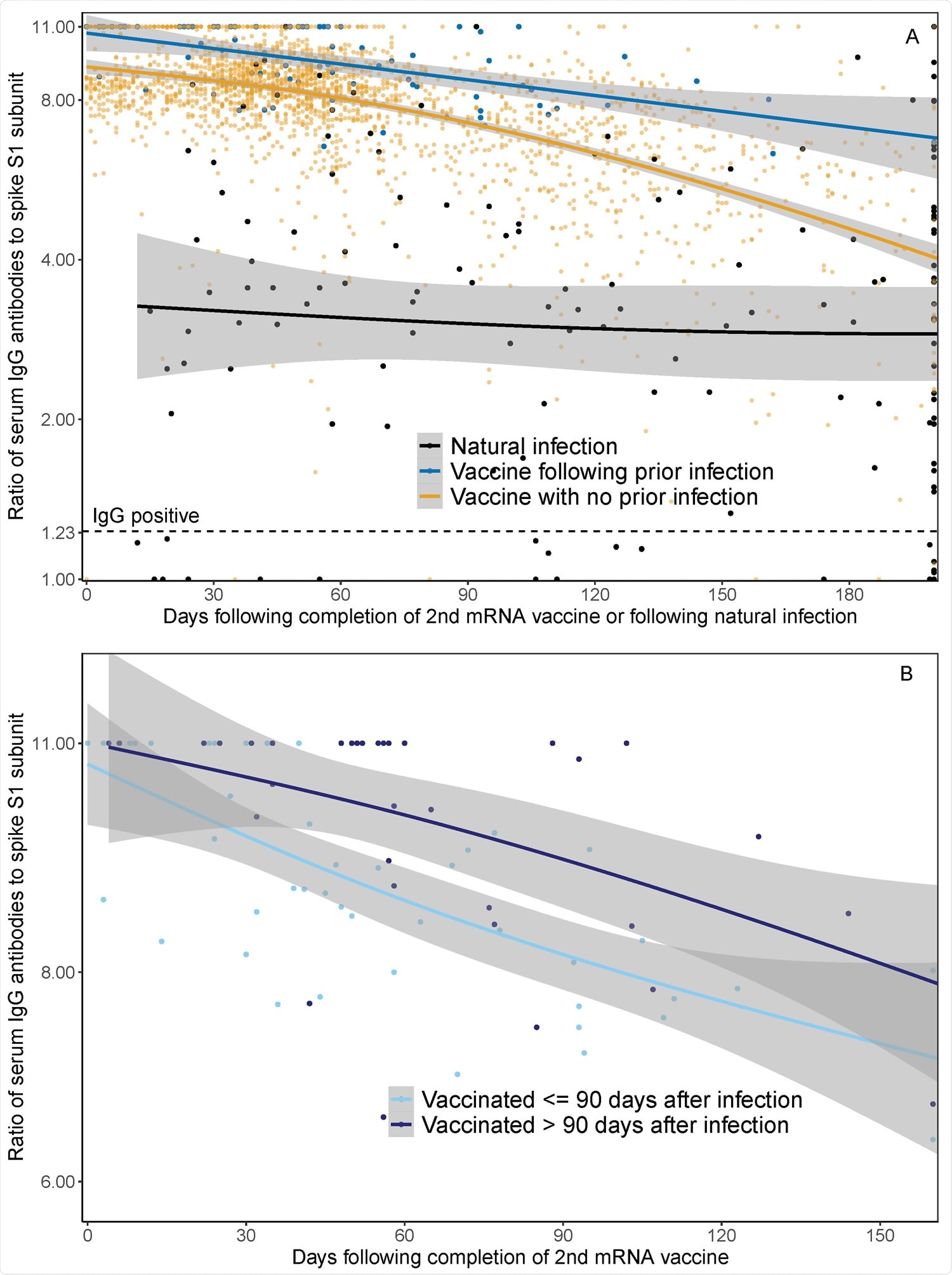
Waning IgG antibodies to SARS-CoV-2 following infection and vaccination in HWs: Panel A demonstrates serum spike S1 IgG antibody measurements in healthcare workers ≥ 14 days following dose two of SARS-CoV-2 mRNA vaccine with prior SARS-CoV-2 infection (blue line), ≥ 14 days following dose two of SARS-CoV-2 mRNA vaccine in those without prior SARSCoV-2 infection (orange line), and in those following SARS-CoV-2 positive PCR test and before vaccination (black line). Prior SARS-CoV-2 infection was defined as positive SARS-CoV-2 PCR prior to first dose of mRNA vaccine. The x-axis represents days of follow-up from infection or vaccination, rather than calendar time, such that 49 HWs contributed to both natural infection and post-vaccination groups, respectively. The lines represent median IgG as a function of days following mRNA vaccination or natural infection, based on natural cubic splines (2 degrees of freedom) for each group. Shaded areas represent 95% Confidence Intervals. Panel B illustrates serum spike S1 IgG antibody decay after mRNA vaccination among participants with SARS-CoV-2 infection ≤ 90 days before vaccination (light blue line) and > 90 days before vaccination (dark blue line). The lines represent median IgG following mRNA vaccination over time using natural cubic splines (2 degrees of freedom). Shaded areas represent 95% Confidence Intervals.
Major findings
- Serum spike IgG Ab levels were higher after vaccination than after natural infection.
- Individuals with prior infection before vaccination continued to have higher serum IgG Ab levels, at 1, 3, and 6 months (following completion of 2nd dose of vaccine, or following natural infection), when compared to individuals with infection alone or those with vaccination alone.
- Individuals with PCR confirmed infection > 90 days before vaccination had higher post-vaccination Ab levels than individuals infected ≤ 90 days before vaccination by approximately 10 percent, as studied at 1 and 3 months.
Longer interval between infection and first vaccine dose enhances Ab response
In agreement with another study comparing extended vaccine dosing intervals, the authors suggest a longer interval of at least 90 days between infection and first vaccine dose (second exposure) for enhanced Ab response. Subsequently, the booster dose which provides a third exposure induces a more durable Ab response.
"Vaccination after natural infection reduces risk of reinfection," the team concludes.
The researchers also highlight the need to inquire whether the improved post-vaccination Ab durability in prior infected individuals is attributable to the number of exposures, the interval between exposures, or a complex interplay between natural and vaccine-derived immunity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zhong D., et al. (2021). Impact of prior SARS-CoV-2 infection on post-vaccination SARS-CoV-2 spike IgG antibodies in a longitudinal cohort of healthcare workers. medRxiv preprint server. doi: https://doi.org/10.1101/2021.09.16.21263576, https://www.medrxiv.org/content/10.1101/2021.09.16.21263576v1
- Peer reviewed and published scientific report.
Zhong, Diana, Shaoming Xiao, Amanda K. Debes, Emily R. Egbert, Patrizio Caturegli, Elizabeth Colantuoni, and Aaron M. Milstone. 2021. “Durability of Antibody Levels after Vaccination with MRNA SARS-CoV-2 Vaccine in Individuals with or without Prior Infection.” JAMA, November. https://doi.org/10.1001/jama.2021.19996. https://jamanetwork.com/journals/jama/fullarticle/2785919.