The global scientific community was able to come together to develop an effective vaccine against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) within 11 months of the World Health Organization (WHO) declaring the coronavirus disease (COVID19) as a pandemic. The primary goal of vaccination efforts has been to reduce rates of severe infection and hospitalizations.
Two vaccines that were initially launched included the Pfizer–BioNTech BNT162b2 and Moderna mRNA-1273 vaccines. Vaccination doses were subsequently approved for administration in many countries around the world from December 2020.
The nation of Qatar experienced two back-to-back waves of SARS-CoV-2 from January through June 2021, which were dominated by the B.1.1.7 (Alpha) and B.1.351 (Beta) SARs-CoV-2 variants. Community transmission of the B.1.617.2 (Delta) SARS-CoV-2 variant was first detected toward the end of March 2021, and quickly became the dominant circulating variant by the summer of 2021.
The continued effectiveness of the Pfizer–BioNTech BNT162b2 vaccine against infection and disease with the dominant B.1.351 (Beta) and B.1.617.2 (Delta) variants in Qatar remains unclear. Despite high vaccine coverage, the incidence of SARS-CoV-2 infection increased slowly from July to August 2021, before starting to decline at the end of August.
The researchers of a new study published in the New England Journal of Medicine assessed the real-world effectiveness of the BNT162b2 vaccine against SARS-CoV-2 infection and COVID-19–related hospitalization and death after receipt of the first and second doses.
About the study
The researchers conducted the current study in the resident population of Qatar. To this end, the researchers obtained all relevant data regarding COVID-19 laboratory testing, vaccination, clinical infection data, and related demographic details from the integrated, nationwide, and verified digital health information platform that hosts the national and federated SARS-CoV-2 databases.
The researchers used a matched test-negative, case-control study design to estimate vaccine effectiveness against any SARS-CoV-2 infection, as well as against any severe, critical, or fatal case of COVID-19 from January 1, 2021, to September 5, 2021.
Demographic data revealed that a total of 947,035 people received at least one dose of the BNT162b2 vaccine, and 907,763 completed the two-dose regimen between December 21, 2020, and September 5, 2021. The median date of the first dose was April 21, 2021, and the median date of the second dose was May 10, 2021.
The median time between the first and second doses was 21 days, with 97.4% of people receiving their second dose within 30 days after the first dose. During this period, 564,196 people received at least one dose of the mRNA-1273 vaccine, with 494,859 completing the two-dose regimen for this vaccine.
Statistical analysis was used to determine the rates of SARS-CoV-2 infection among cohorts divided according to age, sex, time-lapsed from their first and second doses of the vaccine, ethnicity, and reasons for reverse-transcriptase polymerase chain reaction (RT-PCR) testing.
By September 5, 2021, a total of 8,203 SARS-CoV-2 BNT162b2 breakthrough infections (infections despite being vaccinated) had been recorded among participants who received one dose of this vaccine. Comparatively, 10,543 breakthrough infections were reported among participants who received both doses.
The percentage of all daily diagnosed SARS-CoV-2 infections that were vaccine (BNT162b2 or mRNA-1273) breakthrough infections increased gradually over time and reached 36.4% on September 5, 2021. Most vaccine breakthrough infections (77.2%) were recorded for the BNT162b2 vaccine.
The estimated effectiveness of the BNT162b2 vaccine against any SARS-CoV-2 infection was negligible in the first 2 weeks after the first dose. This effectiveness increased to 36.8% in the third week after the first dose and reached its peak at 77.5% in the first month after the second dose.
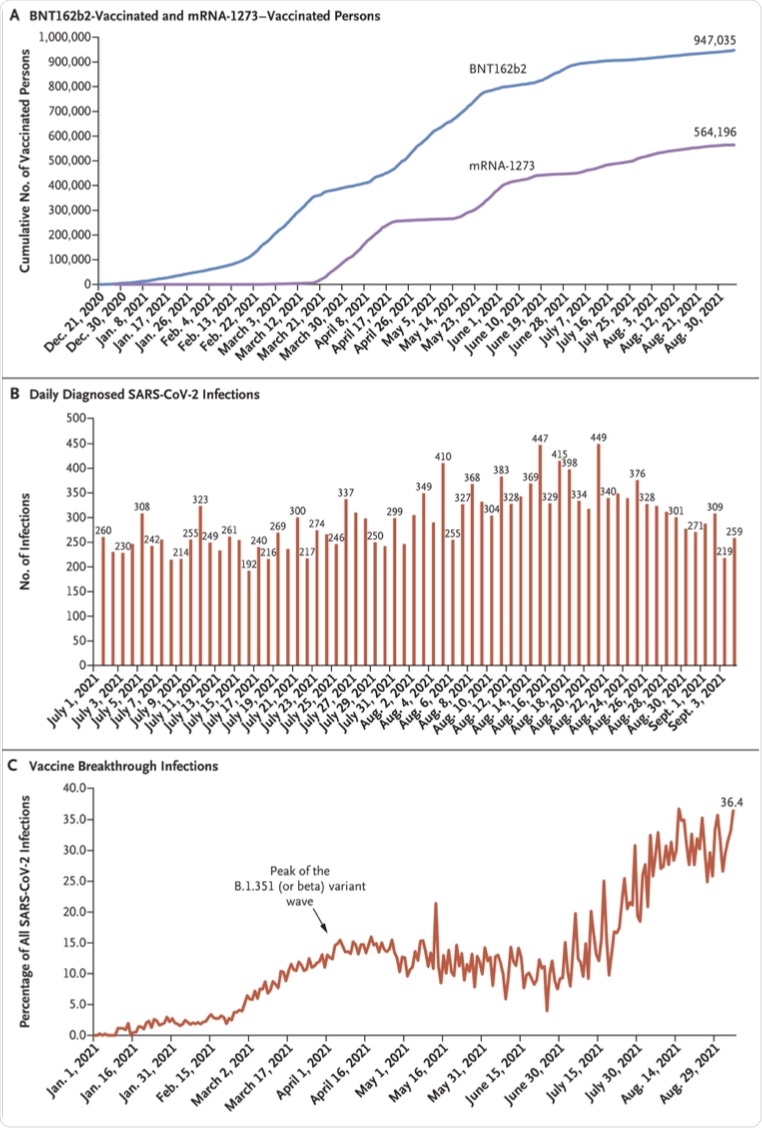 Time Trend of Vaccinated Persons, SARS-CoV-2 Infections, and Vaccine Breakthrough Infections in Qatar.
Time Trend of Vaccinated Persons, SARS-CoV-2 Infections, and Vaccine Breakthrough Infections in Qatar.
Vaccine effectiveness declined gradually thereafter, with the decline accelerating after the fourth month to reach approximately 20% in months 5 through 7 after the second dose. Effectiveness against symptomatic infection was consistently higher at 81.5%, whereas that against asymptomatic infection was 73.1%; however, both waned similarly.
Variant-specific effectiveness waned in the same pattern. Effectiveness against any severe, critical, or fatal case of COVID-19 increased rapidly to 66.1% by the third week after the first dose and reached 96% or higher in the first 2 months after the second dose. Taken together, vaccine effectiveness persisted at approximately this level for 6 months.
Study implications
The current study found that BNT162b2-induced protection against severe SARS-COV-2 infection and hospitalization lasted for 6 months after the second dose. However, its effectiveness declined rapidly following its peak after the second dose.
The results of the current study are crucial in determining the course of vaccination and booster doses in Qatar in an effort to control severe infections and contributing to COVID-19 mortality.
Journal reference:
- Chemaitelly, H., Tang, P., Hasa, M., et al. (2021). Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. The New England Journal of Medicine. doi:10.1056/NEJM0a2114114.