Researchers have shown that the number of prescriptions filled out for a treatment associated with improved pregnancy outcomes among individuals with substance use disorder declined in the United States as the coronavirus disease 2019 (COVID-19) pandemic continued.
Prior to the COVID-19 pandemic, there were barriers to accessing the combination drug – buprenorphine/naloxone – during pregnancy, but Ashley O’Donoghue and colleagues from the Beth Israel Deaconess Medical Center in Boston, Massachusetts, suspected that disruptions to healthcare services during the pandemic may have further exacerbated these barriers.
Using linked national pharmacy claims and medical claims data from May 2019 to December 2020, the researchers have shown that the number of pregnant individuals filling prescriptions for the treatment increased prior to the pandemic. However, this growth was lost during the pandemic across both patients with commercial insurance and those on Medicaid, says the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The prenatal use of medication for opioid use disorder
The number of pregnant individuals with opioid use disorder (OUD) has increased four-fold over the last decade. The prenatal use of medication for OUD such as buprenorphine/naloxone is associated with improved adherence to prenatal care and pregnancy outcomes such as lower rates of preterm birth and low birth weight and lower rates of maternal relapse and overdose.
Despite this, only 55% of pregnant women with OUD were on medication for their substance use disorder prior to the COVID-19 pandemic. This could have been due to the multiple barriers to accessing the treatment, including stigma, fear of legal or child welfare outcomes, and limited access to obstetric and addiction treatment experts.
To help address any potential loss of care access during the COVID-19 pandemic, the federal government permitted the initiation of buprenorphine/naloxone treatment via telehealth in June 2020.
Pregnancy provides a crucial opportunity to help women with OUD, but it is not clear how the pandemic affected access to treatment in this population.
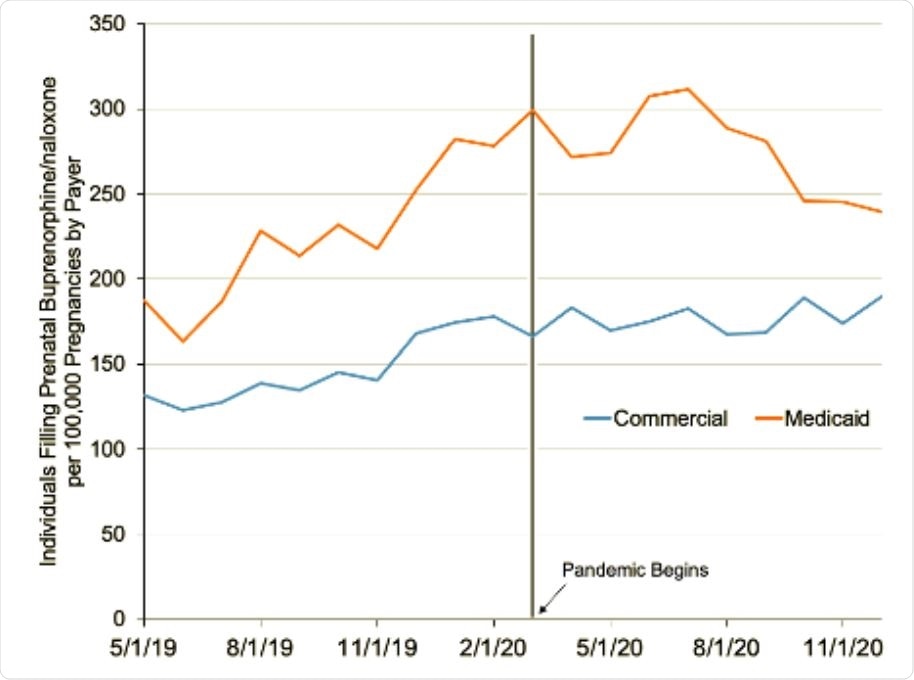
Trends in individuals filling prenatal buprenorphine/naloxone prescriptions each month from Symphony Health claims data from May 2019 to December 2020. The y-axis represents the number of individuals filling prenatal buprenorphine/naloxone prescriptions, weighted by the total number of pregnancies for a given month and payer. This weighting is to account for any seasonality in pregnancies or changing trends in pregnancies during the pandemic that may differ over time and by payer. The vertical black line denotes March 2020, when the pandemic began in the United States.
What did the researchers do?
O’Donoghue and colleagues applied an interrupted time series design to examine monthly trends in the number of pregnant patients filling prescriptions for prenatal buprenorphine/naloxone before and during the COVID-19 pandemic.
They used linked national pharmacy claims and medical claims data from May 2019 to December 2020 and defined prenatal buprenorphine/naloxone prescription fills as those filled in the six months prior to delivery.
The researchers weighted the monthly count of prenatal prescription fills for Medicaid patients and commercially insured patients by the total monthly number of pregnancies for that payer. The pre-pandemic (May 2019 to February 2020) level and growth rate were compared to the post-pandemic (April 2020 to December 2020) level and growth rate of the number of individuals filling these prescriptions.
What did the study find?
The study identified 2,947 pregnant patients filling the prescriptions. More than half (55.5%) of these patients were aged 21 to 30 years and most (58.1%) were on commercial insurance, with 38.9% on Medicaid.
Prior to the COVID-19 pandemic, the monthly growth rate in the number of individuals filling the prescriptions was 4.83% across all payers (5.35% among those on Medicaid and 4.06% for those on commercial insurance).
At the beginning of the pandemic, there was no immediate statistically significant change in the number of individuals filling the prescriptions. However, the monthly growth rate declined by 5.53% across all payers as the pandemic continued, falling by 7.66% among those on Medicaid and by 3.59% among those on commercial insurance.
The researchers say the findings suggest that prior to the COVID-19 pandemic, the number of pregnant individuals filling buprenorphine/naloxone prescriptions was increasing.
“This growth, however, has been lost during the pandemic, across both patients on commercial insurance and Medicaid,” concludes the team.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.