A recent research paper by scientists from Denmark reveals that circulating plasmacytoid dendritic cells may be a potential therapeutic target to maintain desired levels of an antiviral compound known as interferon, allowing for the mitigation of coronavirus disease (COVID-19) severity. The study is currently available on the bioRxiv* preprint server while it undergoes peer review.
Since the start of the COVID-19 pandemic, many studies have hinted that different cell types (but also diverging sensing pathways) may be responsible for controlling viral infection, but also for the surge in inflammatory cytokines that are characteristic for the infected patients.
In our immune system, plasmacytoid dendritic cells are autonomous producers of type I interferon-alpha, making them one of the key players in controlling viral infections. Clinical studies have shown that severe COVID-19 cases have a reduction in circulating plasmacytoid dendritic cells, as well as minimal influx of these cells into the lungs (when compared to patients with moderate forms of the disease and healthy controls).
However, it is unclear whether disease severity arises due to the lack of plasmacytoid dendritic cells in the lungs or as a result of dysfunctional cytokine production. In addition, the mechanism of how these cells may sense SARS-CoV-2 has not been determined.
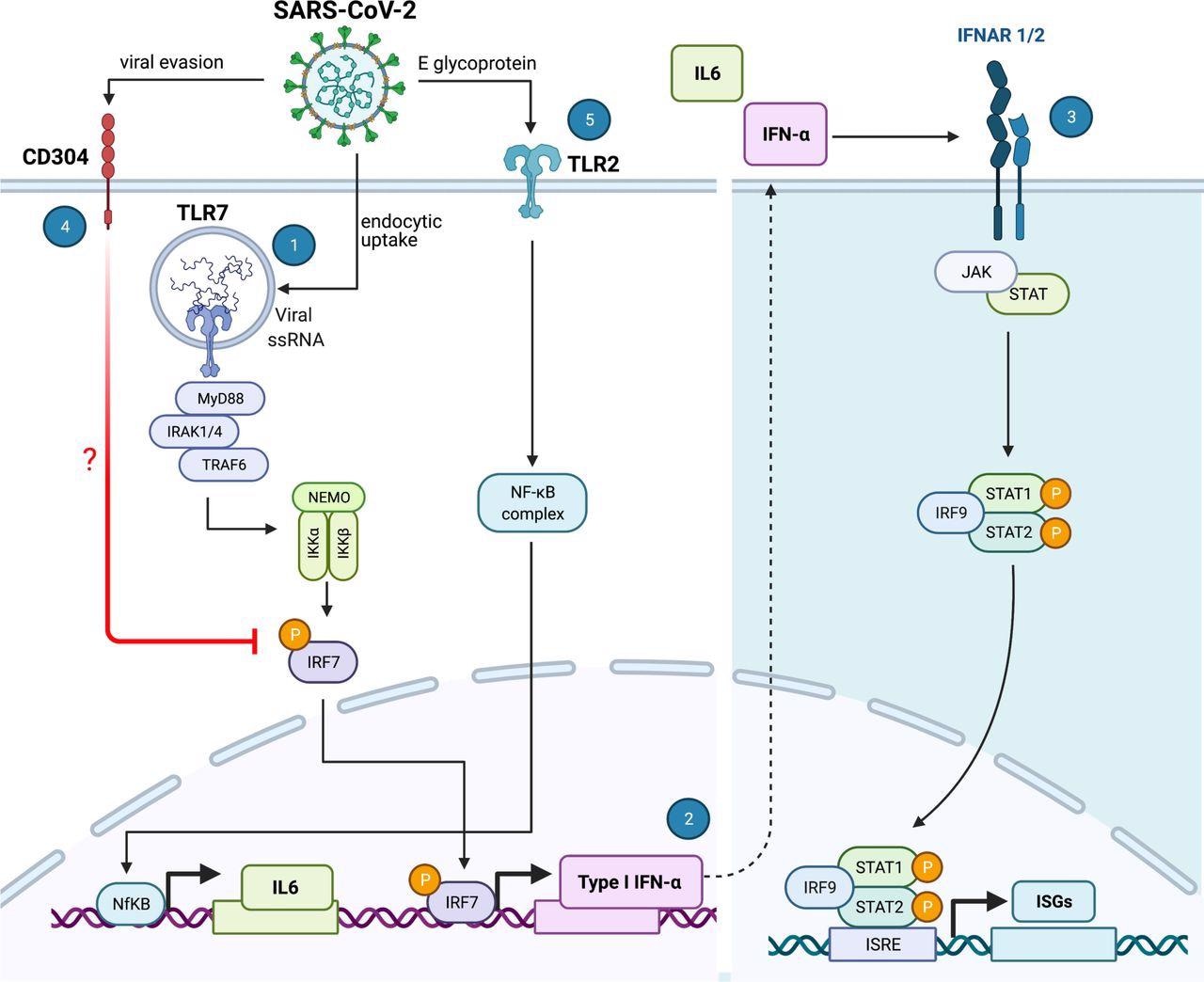 SARS-CoV-2 sensing by plasmacytoid dendritic cells. SARS-CoV-2 is internalized by pDCs via a yet unknown endocytic mechanism. The intracellular TLR7 sensor detects viral RNA and induces a signaling cascade involving MyD88-IRAK4-TRAF6 (1) to induce CXCL10 and, via IRF7 phosphorylation and translocation, inducing type I and III Interferons (2). Once secreted, type I and III IFNs initiate autocrine and paracrine signals that induce the expression of IFN stimulated genes (ISGs), thereby facilitating an antiviral response that can protect the cell against infection. However, SARS-CoV-2 has the intrinsic property to facilitate CD304 signaling, potentially by interfering with IRF7 nuclear translocation, thereby inhibiting type I IFNα production and thus reducing the antiviral response generated by the pDC (4). Furthermore, the SARS-CoV-2 envelope (E) glycoprotein is sensed by the extracellular TLR2/6 heterodimer and this facilitates the production of the inflammatory IL-6 cytokine (5). The illustration was created with BioRender.com
SARS-CoV-2 sensing by plasmacytoid dendritic cells. SARS-CoV-2 is internalized by pDCs via a yet unknown endocytic mechanism. The intracellular TLR7 sensor detects viral RNA and induces a signaling cascade involving MyD88-IRAK4-TRAF6 (1) to induce CXCL10 and, via IRF7 phosphorylation and translocation, inducing type I and III Interferons (2). Once secreted, type I and III IFNs initiate autocrine and paracrine signals that induce the expression of IFN stimulated genes (ISGs), thereby facilitating an antiviral response that can protect the cell against infection. However, SARS-CoV-2 has the intrinsic property to facilitate CD304 signaling, potentially by interfering with IRF7 nuclear translocation, thereby inhibiting type I IFNα production and thus reducing the antiviral response generated by the pDC (4). Furthermore, the SARS-CoV-2 envelope (E) glycoprotein is sensed by the extracellular TLR2/6 heterodimer and this facilitates the production of the inflammatory IL-6 cytokine (5). The illustration was created with BioRender.com
Screening via CRISPR-editing approach
The study, first-authored by Renée M. van der Sluis from the Aarhus University in Denmark, aimed to explore the exact molecular mechanism that plasmacytoid dendritic cells utilize in order to sense SARS-CoV-2 once the virus enters a human body.
The research group used the CRISPR gene editing approach to screen for several innate immune sensor pathways that are implicated in the production of antiviral interferons and inflammatory cytokines upon viral sensing. For that purpose, blood samples from patients hospitalized due to COVID-19 were collected at hospital admission.
The investigation of sensing mechanisms has been done using a cellular platform designed to generate human primary plasmacytoid dendritic cells ex vivo with the help of hematopoietic stem and progenitor cells from healthy individuals.
Furthermore, a broad exploration of the timing and nature of SARS-CoV-2-induced antiviral responses in plasmacytoid dendritic cells was done by profiling 789 selected genes (covering major immunological pathways) with the use of the NanoString nCounter technology (i.e., a system for digitally detecting and enumerating large sets of molecules).
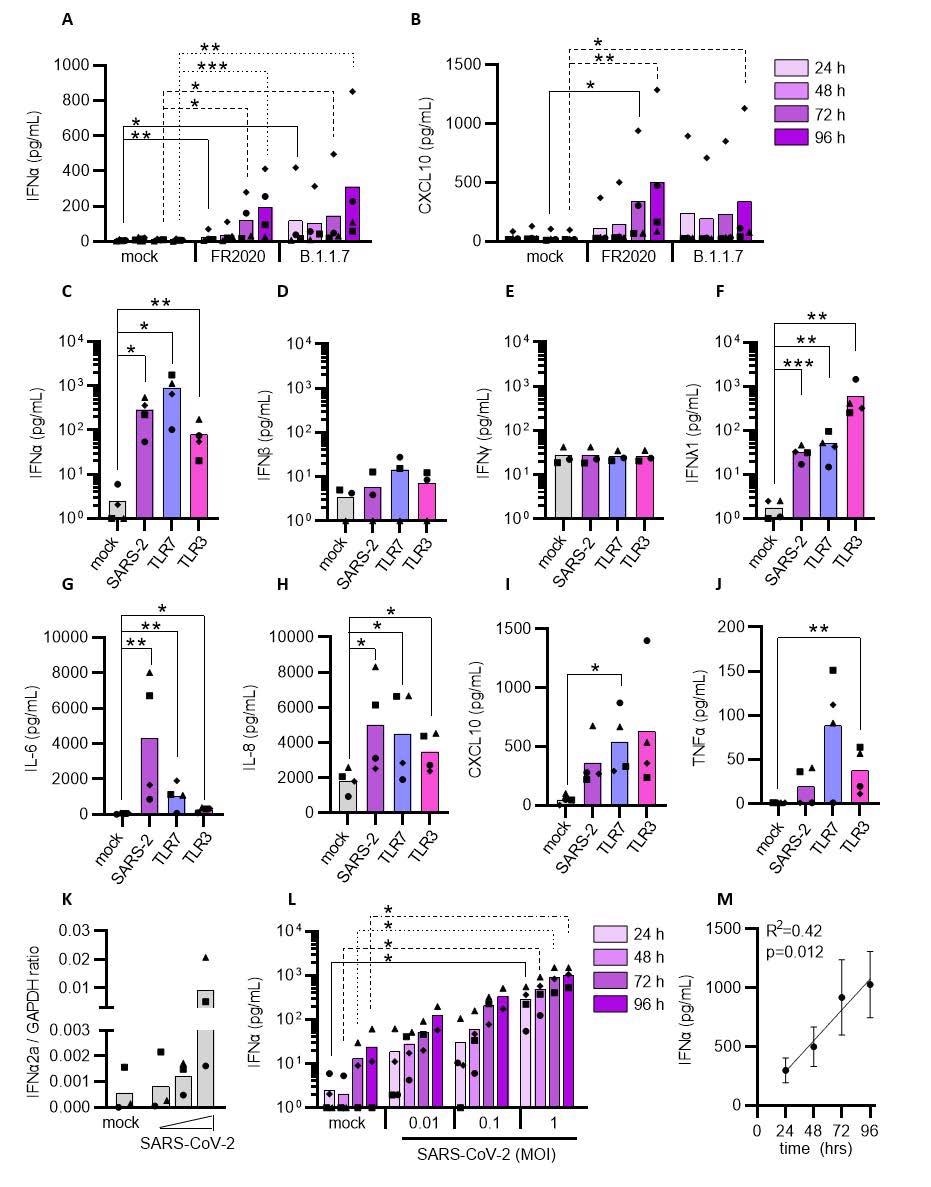 Plasmacytoid DCs can sense SARS-CoV-2 and induce an inflammatory response. pDCs were either mock-treated or exposed to the SARS-CoV-2 FR2020 early Wuhan-like strain or the SARS-CoV-2 alpha variant B.1.1.7 (0.1 MOI). Supernatants were collected at indicated time points and the production of type I IFNα (A) and CXCL10 (B) was quantified. The FR2020 strain was used in subsequent experiments where pDC were either mock-treated (mock, grey), exposed to SARS-CoV-2 at 1 MOI (SARS-2, purple), TLR7 (2.5 μg/mL R837, blue) or TLR3 agonist (800 ng/mL poly(I:C), pink). Supernatants were collected after 24 hrs and analyzed for type I IFNα (C), IFNβ (D), type II IFNγ (E), type III IFNλ1 (F), IL-6 (G), IL-8 (H), CXCL10 (I) and TNFα (J) expression by ELISA. To evaluate the cytokine response to viral titers and exposure duration, pDCs were exposed to increasing viral inoculums (MOI of 0.01, 0.1 and 1) and IFNα2a mRNA expression was quantified at 24 hrs (K) and IFNα protein secretion at 24, 48, 72 and 96 hrs (L). Graph depicting simple linear regression of IFNα protein with time of exposure (M). Bars and lines represent mean values and symbols represent individual pDC donors (n=3-4). Equal symbols represent equal donors (A-B and C-L). Statistical significance was determined using the ratio paired student T test and compared the treated condition with the time point-matched mock condition (A-L) and simple linear regression (M). *
Plasmacytoid DCs can sense SARS-CoV-2 and induce an inflammatory response. pDCs were either mock-treated or exposed to the SARS-CoV-2 FR2020 early Wuhan-like strain or the SARS-CoV-2 alpha variant B.1.1.7 (0.1 MOI). Supernatants were collected at indicated time points and the production of type I IFNα (A) and CXCL10 (B) was quantified. The FR2020 strain was used in subsequent experiments where pDC were either mock-treated (mock, grey), exposed to SARS-CoV-2 at 1 MOI (SARS-2, purple), TLR7 (2.5 μg/mL R837, blue) or TLR3 agonist (800 ng/mL poly(I:C), pink). Supernatants were collected after 24 hrs and analyzed for type I IFNα (C), IFNβ (D), type II IFNγ (E), type III IFNλ1 (F), IL-6 (G), IL-8 (H), CXCL10 (I) and TNFα (J) expression by ELISA. To evaluate the cytokine response to viral titers and exposure duration, pDCs were exposed to increasing viral inoculums (MOI of 0.01, 0.1 and 1) and IFNα2a mRNA expression was quantified at 24 hrs (K) and IFNα protein secretion at 24, 48, 72 and 96 hrs (L). Graph depicting simple linear regression of IFNα protein with time of exposure (M). Bars and lines represent mean values and symbols represent individual pDC donors (n=3-4). Equal symbols represent equal donors (A-B and C-L). Statistical significance was determined using the ratio paired student T test and compared the treated condition with the time point-matched mock condition (A-L) and simple linear regression (M). *
Sensing SARS-CoV-2 and producing cytokines
In short, the researchers have shown that plasmacytoid dendritic cells are capable of sensing SARS-CoV-2 and, in response, produce type I interferon-alpha, prompting, in turn, the production of inflammatory cytokines that give rise to the cytokine storm observed in people suffering from severe forms of COVID-19.
More specifically, plasmacytoid dendritic cells sense SARS-CoV-2 and elicit antiviral protection of lung epithelial cells through Toll-like receptor 7 (TLR7), while recognition of Toll-like receptor 2 (TLR2) elicits an IL-6 inflammatory response associated with immune pathology.
Furthermore, this study emphasizes that SARS-CoV-2 utilizes neuropilin-1 not only as an alternative receptor to the angiotensin-converting enzyme 2 (ACE2) for viral entry but also to mitigate the production of type I interferon-alpha by plasmacytoid dendritic cells – reducing the host’s innate antiviral immune response.
A potential treatment target
The results of this study highlight distinct sensing pathways used by plasmacytoid dendritic cells to prompt antiviral vs. immunopathological responses to SARS-CoV-2 and suggest that targeting neuropilin-1 may be clinically relevant for mounting TLR7-mediated antiviral protection.
“Here we show that in COVID-19 patients, circulating plasmacytoid dendritic cells decline early after symptom onset and this correlated with COVID-19 disease severity”, emphasize study authors in this bioRxiv paper.
In conclusion, this study provides evidence that these circulating could be a potentially promising treatment target that will preserve desired antiviral interferon levels and allow for the mitigation of COVID-19 severity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
van der Sluis, R.M. et al. (2021). Distinct SARS-CoV-2 sensing pathways in pDCs driving TLR7-antiviral vs. TLR2-immunopathological responses in COVID-19. bioRxiv. https://doi.org/10.1101/2021.11.23.469755, https://www.biorxiv.org/content/10.1101/2021.11.23.469755v1
- Peer reviewed and published scientific report.
Sluis, Renée M van der, Lamin B Cham, Albert Gris‐Oliver, Kristine R Gammelgaard, Jesper G Pedersen, Manja Idorn, Ulvi Ahmadov, et al. 2022. “TLR2 and TLR7 Mediate Distinct Immunopathological and Antiviral Plasmacytoid Dendritic Cell Responses to SARS‐CoV‐2 Infection.” The EMBO Journal, March. https://doi.org/10.15252/embj.2021109622. https://www.embopress.org/doi/full/10.15252/embj.2021109622.