In a recent study posted to the bioRxiv* pre-print server, an international team of researchers reported the occurrence of coronavirus disease 2019 (COVID-19) breakthrough infections (BTIs) among health care workers (HCWs) participating in a phase III vaccine trial in Sisonke, South Africa.
 Study: Breakthrough Covid-19 infections during periods of circulating Beta, Delta and Omicron variants of concern, among health care workers in the Sisonke Ad26.COV2.S vaccine trial, South Africa. Image Credit: Fit Ztudio
Study: Breakthrough Covid-19 infections during periods of circulating Beta, Delta and Omicron variants of concern, among health care workers in the Sisonke Ad26.COV2.S vaccine trial, South Africa. Image Credit: Fit Ztudio

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant was declared a variant of concern (VOC) on 26 Nov 2021 by the World Health Organization (WHO) and was first identified in South Africa. While there are experimental and epidemiological data to support immune escape by Omicron in previously infected and vaccinated individuals and higher re-infection rates with Omicron, compared to the Beta and Delta VOCs, the data on the severity of Omicron-driven breakthrough infections (BTIs) is scarce. However, positive COVID-19 reverse transcriptase-polymerase chain reaction (RT-PCR) or antigen tests at or after 28 days post-vaccination confirms a BTI occurrence.
The study
From 17 Feb to 15 Dec 2021, the researchers gathered BTI frequency and severity-related data for the periods in which the Beta (17 February-17 May 2021 (89 days)), Delta (18 May-14 November 2021 (180 days)), and Omicron (15 November-15 December 2021 (30 days)) VOCs were circulating.
The HCWs who consented to participate in the study worked at 350 Sisonke vaccine trial vaccination centers, spread across nine South African provinces. The National Electronic Vaccination Data System (EVDS) provided information on the vaccination records to monitor BTIs in the participating HCWs. All the vaccinated HCWs were notified via text messages to report BTIs telephonically or via a web link, and hospitalized HCWs reported disease severity and comorbidities to a post-vaccination safety monitoring team.
Results
Over 40,000 BTIs were observed during the study period, of which 609 occurred during the Beta period, 22,279 during the Delta period, and 17,650 during the Omicron period. By 15 Dec 2021 (end of the study period), Omicron-induced daily BTIs increased to up to three times compared to the peak observed during the Delta period. Compared to the Delta variant, Omicron infected more HCWs in the 18-30 year age group and fewer individuals over 55 years.
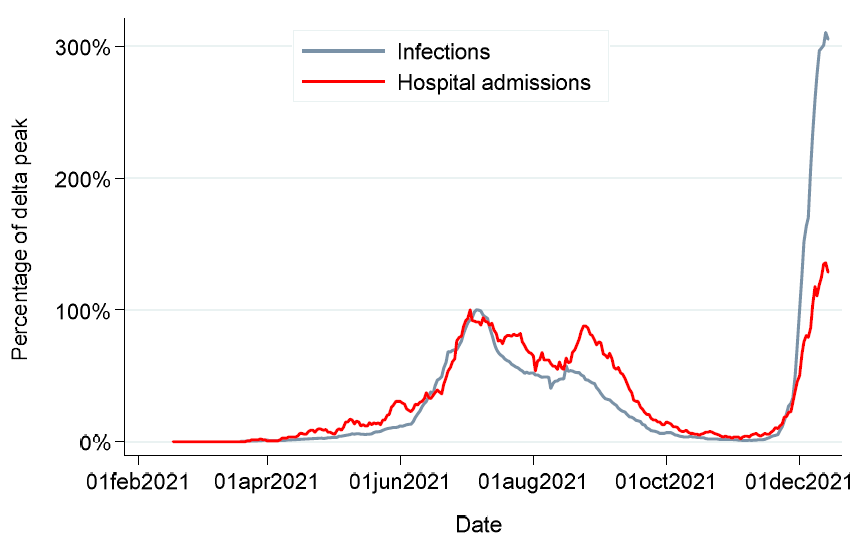
Daily cases and admissions expressed as a proportion of delta peak
There were 1,914 BTI-related hospitalizations - 77, 1429, and 408 in the Beta, Delta, and Omicron periods, respectively. The HCWs in the 31-54 age group showed increasing proportions of BTI-related hospitalizations, from 51% during Beta to 60% during Delta and 67% during the Omicron periods. In HCWs over 55 years of age, BTI-related hospitalizations reduced from 46% during the Beta to 19% during the Omicron period.
During the Omicron period, 3% of hospitalized HCWs required intensive care, which was significantly lower than 7% and 16% during the Delta and Beta periods, respectively. Furthermore, the median length of hospitalization among HCWs was six days for Beta, five days for Delta, and three days for Omicron.
Of the 17,650 BTIs which occurred during the Omicron period, 2% suffered primary infections during the Beta period compared to 5% during the Delta period, indicating some cross-protection induced by prior-infection by the Beta variant. Interestingly, these re-infected HCWs (previously infected during Beta/Delta periods) showed no significant differences in baseline characteristics, such as age, sex, and comorbidities.
Conclusions
The study findings are based on the first 30-days of the Omicron period and illustrate more BTIs during the Omicron period than that seen during the Delta period peak, however, less severe.
This study has several limitations, such as the non-applicability of its findings to all settings and populations globally. Also, the study does not account for person-time follow-up and presents BTI incidences only during specific time periods when VOCs were circulating. While the study includes data of BTIs needing COVID-19-related hospitalization across all study periods, including hospitalizations for reasons like surgery might have resulted in over-estimation of the numbers and proportions of BTIs.
Despite these limitations, the large cohort of the study produced enough evidence to chart an early snapshot of the Omicron’s effect in a high SARS-CoV-2 seroprevalence setting.
The authors plan to update these findings over time and as more BTIs ensue in this cohort. However, future studies should appropriately investigate the reason(s) for increased Omicron-driven primary infections and re-infections - increased Omicron infectivity, Omicron immune evasion, or a combination of all of these factors.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Breakthrough Covid-19 infections during periods of circulating Beta, Delta, and Omicron variants of concern, among health care workers in the Sisonke Ad26.COV2.S vaccine trial, South Africa, Ameena Ebrahim Goga, Linda-GAIL Bekker, Nigel Garret, Tarylee Reddy, Nonhlanhla Yende-Zuma, Lara Fairall, Harry Moultrie, Azwidihwi Takalani, Valentina Trivelli, Mark Faesen, Veronique Baley, Ishen Seocharan, Glenda Elisabeth Gray, medRxiv (2021), 12.21.21268171, doi: https://doi.org/10.1101/2021.12.21.21268171, https://www.medrxiv.org/content/10.1101/2021.12.21.21268171v2
- Peer reviewed and published scientific report.
Goga, Ameena, Linda-Gail Bekker, Nigel Garrett, Tarylee Reddy, Nonhlanhla Yende-Zuma, Lara Fairall, Harry Moultrie, et al. 2022. “Breakthrough SARS-CoV-2 Infections during Periods of Delta and Omicron Predominance, South Africa.” The Lancet 400 (10348): 269–71. https://doi.org/10.1016/s0140-6736(22)01190-4. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)01190-4/fulltext.