
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Several coronavirus disease 2019 (COVID-19) vaccines have been granted emergency use authorization (EUA) in many countries around the world. Some of these vaccines include the Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273 vaccines, both of which have been highly effective against SARS-CoV-2 infection.
In addition to vaccination, MAb cocktails from Regeneron consisting of REGN10933 (Casirivamab) and REGN10987 (Imdevimab), as well as those produced by Eli Lilly consisting of LY-CoV016 (Etesevimab) and LY-CoV555 (Bamlanivimab) have substantially decreased infection rates and COVID-19-related hospitalization and deaths.
However, the newly emerged SARS-CoV-2 Omicron (B.1.1.529) variant of concern (VOC) is likely unfazed by the effectiveness of currently approved COVID-19 vaccines and MAbs. This is largely due to the fact that the Omicron variant has 34 mutations in its spike (S) protein, 20 of which have never been seen in previous VOCs or variants of interests (VOIs).
The 15 mutations in the Omicron receptor-binding domain (RBD), as well as the eight in the amino-terminal domain (NTD) and 10 in the carboxy-terminal (CTD), are the most concerning mutations, all of which are the primary sites targeted by neutralizing antibodies (NAbs).
About the study
In the current study, researchers collected human sera samples from consenting participants at the New York University (NYU) Vaccine Center in the United States. Convalescent sera and sera from BNT162b2 and mRNA-1273-vaccinated participants were collected 32-57 days post-symptom onset, and 90 and 80 days mean post-second immunization, respectively.
Serum samples from participants previously infected and subsequently vaccinated with the BNT162b2 messenger ribonucleic acid (mRNA) vaccine were collected one month and seven-eight months post-second immunization. Sera were also collected from individuals vaccinated with the BNT162b2 booster dose one-month post-vaccination.
The researchers measured the neutralizing antibody titers in sera from both naïve and convalescent study participants and also analyzed the neutralizing activity of several widely used therapeutic MAbs. The expression vector pc.Δ19.Omicron encoding SARS-CoV-2 Omicron S proteins were chemically synthesized. Further, individual S mutations were generated by polymerase chain reaction (PCR) mutagenesis using the D614G S protein plasmid pcCOV2.Δ19.D614G as a template.
Study findings
Although the sera of unvaccinated participants showed moderately reduced antibody titers against the SARS-CoV-2 Beta and Delta variants as compared to the parental D614G, there was an average 26-fold reduction in titers against the Omicron variant.
Sera collected 70 days post-immunization from study participants who received two doses of BNT162b2 or mRNA-1273 vaccines showed 3-4-fold higher titers against the D614G strain as compared to convalescent sera. However, antibody titers against the Omicron variant decreased 26-34-fold compared to D614G.
One-month after the participants had received their second vaccine dose who had not experienced a previous infection had high titers against the D614G strain. Comparatively, titers against the Beta, Delta, and Omicron variants decreased 2.8-fold, 1.4-fold, and 18-fold, respectively.
Neutralizing antibody titers were also found to decline 7-8 months post-immunization but increased for all variants one-month post-booster dose. The study participants who had poor antibody titers against the Omicron variant after two immunizations had increased their titers following the booster dose; however, titers remained 14-fold lower as compared to when challenged with the D614G strain.
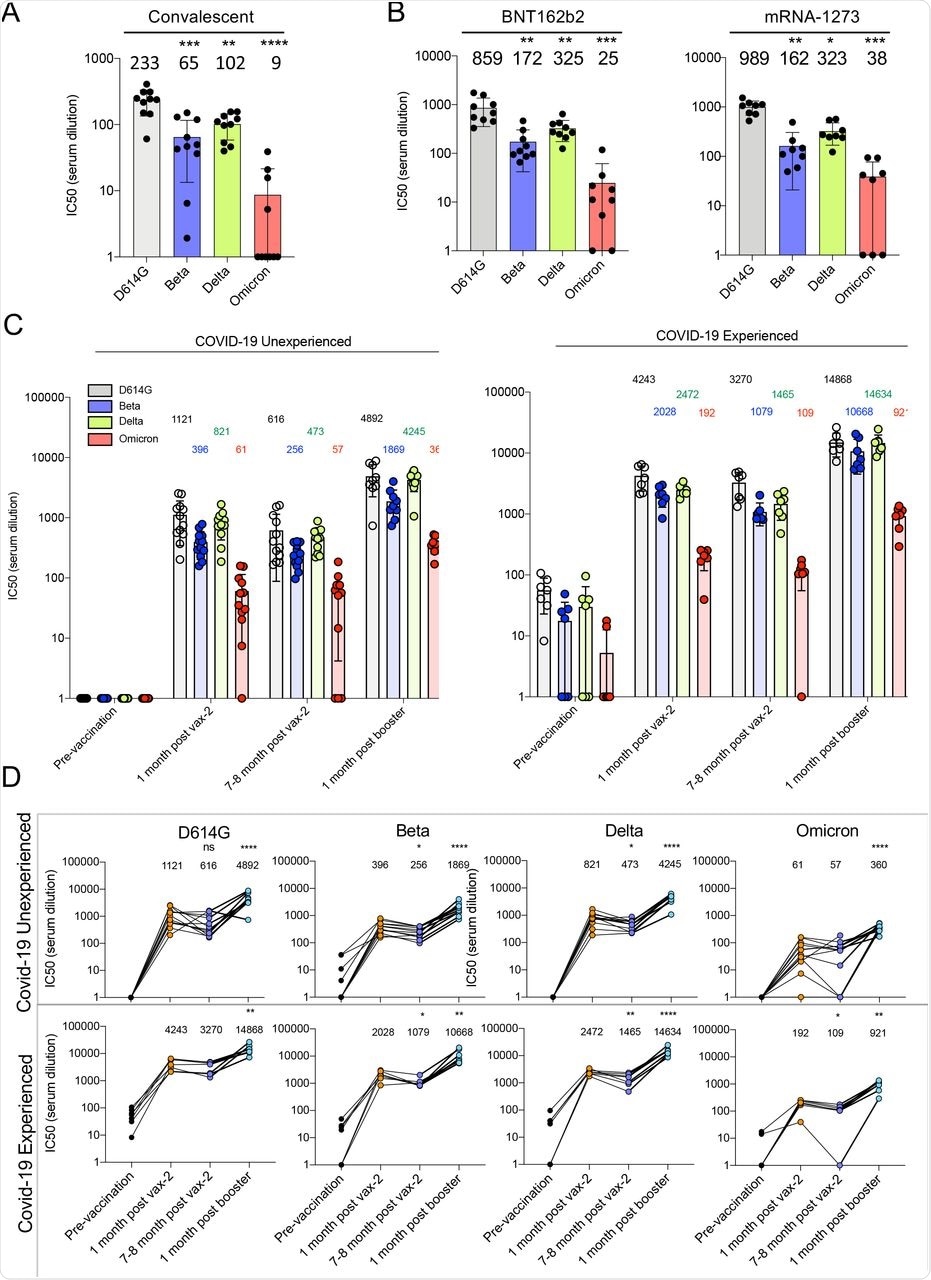
Decreased neutralization of Omicron spike protein-pseudotyped viruses by convalescent sera, mRNA vaccine-elicited antibodies.
While all MAbs and MAb cocktails investigated in this study showed weak or no activity against the Omicron variant, VIR-7831 (Sotrovimab) was active against Omicron with its inhibitory concentration of 50% (IC50) that was about 172-fold lower than against D614G. Although its neutralizing activity decreased in treated patients, VIR-7831 achieved an antibody concentration of 24 μg/ml following a 500 mg dose. It is worth noting that a decreased MAb inhibitory activity increases the IC50.
The neutralizing activity of REGN10933 and REGN10987 was affected by mutations K417N, 217 E484A, Q493K, and S371L, S373P, N440K, G446S. However, their cocktail maintained neutralization potency against the single-point mutated virus.
Bamlanivimab inhibitory activity was abolished by E484A and Q493K mutations, while several other mutations had minor effects. Except for the E484A mutation, most other mutations had moderate effects on neutralizing titers, thereby suggesting that the loss of activity of the MAbs was due to the combined effect of all Omicron S mutations.
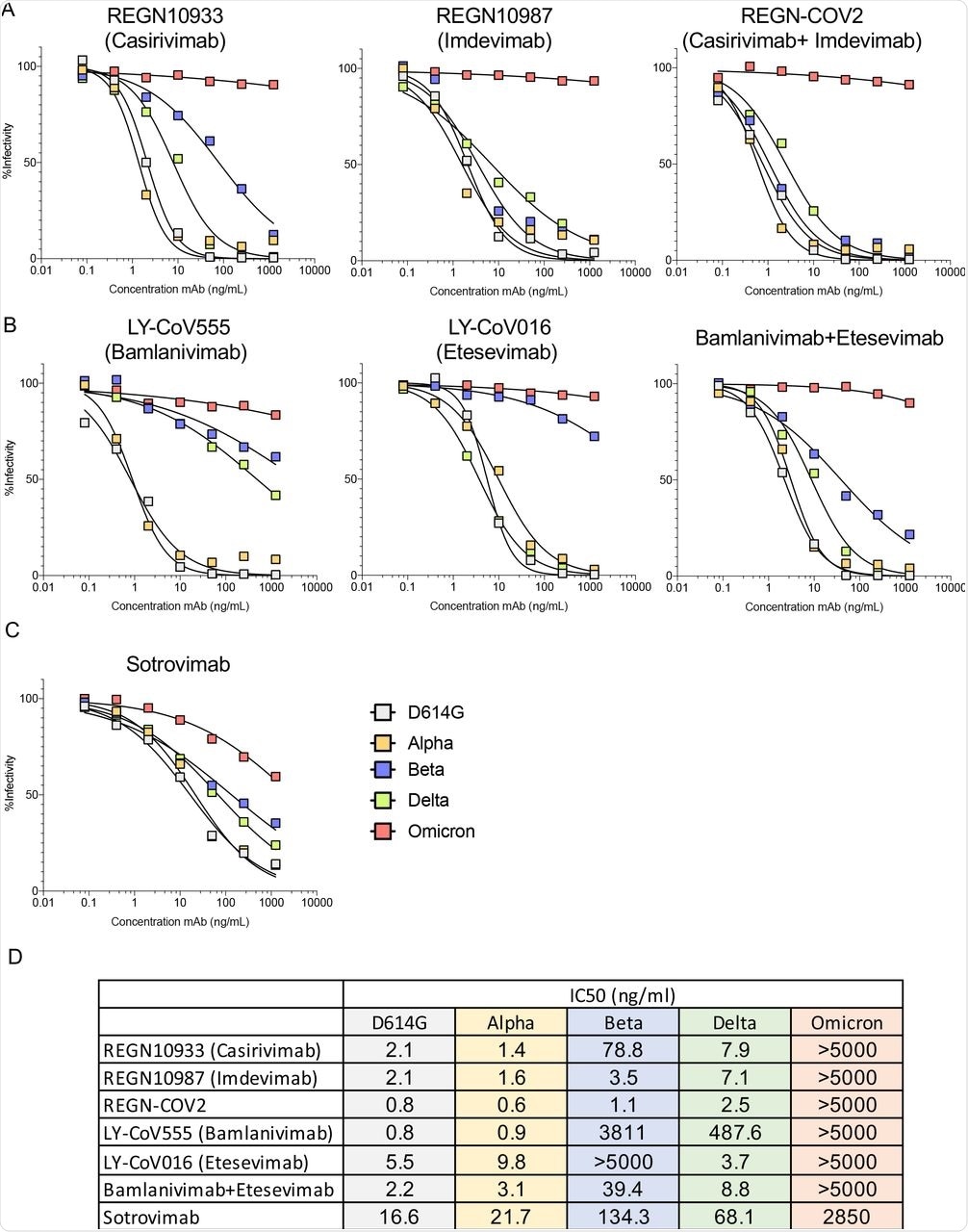
Therapeutic monoclonal antibodies have lost neutralizing activity against virus with the Omicron spike protein.
Conclusions
The current study findings highlight the benefits of booster immunization with an mRNA vaccine and the need to develop more effective MAb therapeutics for COVID-19 treatment.
While the pseudotyped lentivirus with the Omicron S protein showed about 2,640-fold increased resistance, the Alpha, Beta, Gamma, and Delta VOCs showed only 3-4-fold resistance to vaccine-elicited antibodies.
The researchers predicted antibody titers of study participants fully vaccinated with BNT162b2 or Moderna-1273 to cause an increased frequency of breakthrough infections (BTIs). Interestingly, the study findings showed that the BNT162b2 vaccine increased titers against Omicron to highly protective levels, yet the antibody titers remained about 10-fold below those against other VOCs post-booster. Furthermore, the durability of these titer values remains undetermined.
To summarize, the study findings suggest that the frequency of Omicron-induced infections is likely to increase; however, vaccination with two doses followed by a homologous booster immunization will increase antibody titers almost five-fold, thereby providing protection against severe COVID-19. The vaccination-induced T-cell response may also provide additional protection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Tada, T., Zhou, H., Dcosta, B. M., et al. (2021). Increased resistance of SARS-CoV-2 Omicron Variant to Neutralization by Vaccine-Elicited and Therapeutic Antibodies. bioRxiv. doi:10.1101/2021.12.28.474369. https://www.biorxiv.org/content/10.1101/2021.12.28.474369v1.
- Peer reviewed and published scientific report.
Tada, Takuya, Hao Zhou, Belinda M. Dcosta, Marie I. Samanovic, Vidya Chivukula, Ramin S. Herati, Stevan R. Hubbard, Mark J. Mulligan, and Nathaniel R. Landau. 2022. “Increased Resistance of SARS-CoV-2 Omicron Variant to Neutralization by Vaccine-Elicited and Therapeutic Antibodies.” EBioMedicine 78 (April): 103944. https://doi.org/10.1016/j.ebiom.2022.103944. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(22)00128-1/fulltext.