
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
In an attempt to end the coronavirus disease 2019 (COVID-19) pandemic, global scientists and governments worked together to develop and distribute an effective vaccine against SARS-CoV-2. However, the severity of COVID-19 has been aggravated by the continuous mutation of the virus, which could lead to a decrease in the efficacy of current vaccines.
The effect of newly emerging SARS-CoV-2 variants on the efficacy of vaccines remains a concern for global public health. Therefore, to restrict the potential of new variants, the United States Food and Drug Administration (USFDA) sanctioned booster vaccinations for individuals who had completed the primary vaccination series. These individuals were eligible for the booster dose at least six months after receiving the primary dose of the Moderna and Pfizer-BioNTech vaccines or two months after receiving the primary dose of the Johnson & Johnson SARS-CoV-2 vaccine.
About the Study
In the current study, the researchers aimed to compare the variables that characterize patients with severe SARS-CoV-2 infection requiring hospitalization among FV&B and UV patients by evaluating adult patients who required hospitalization due to severe COVID-19.
The researchers compared demographic and health history data of patients who were FV&B with those who were UV and required hospitalization for COVID-19 during the same period of time. They also studied clinical variables in the FV&B population and identified the variables that correlated with the characteristics of patients in the emergency department (ED) with the need for intensive care unit (ICU) admission.
Adult patients 18 years of age or older who required hospitalization and had a discharge diagnosis of active COVID-19 were included in the study. Patients who had COVID-19 during a previous hospitalization and pediatric patients were excluded from the study.
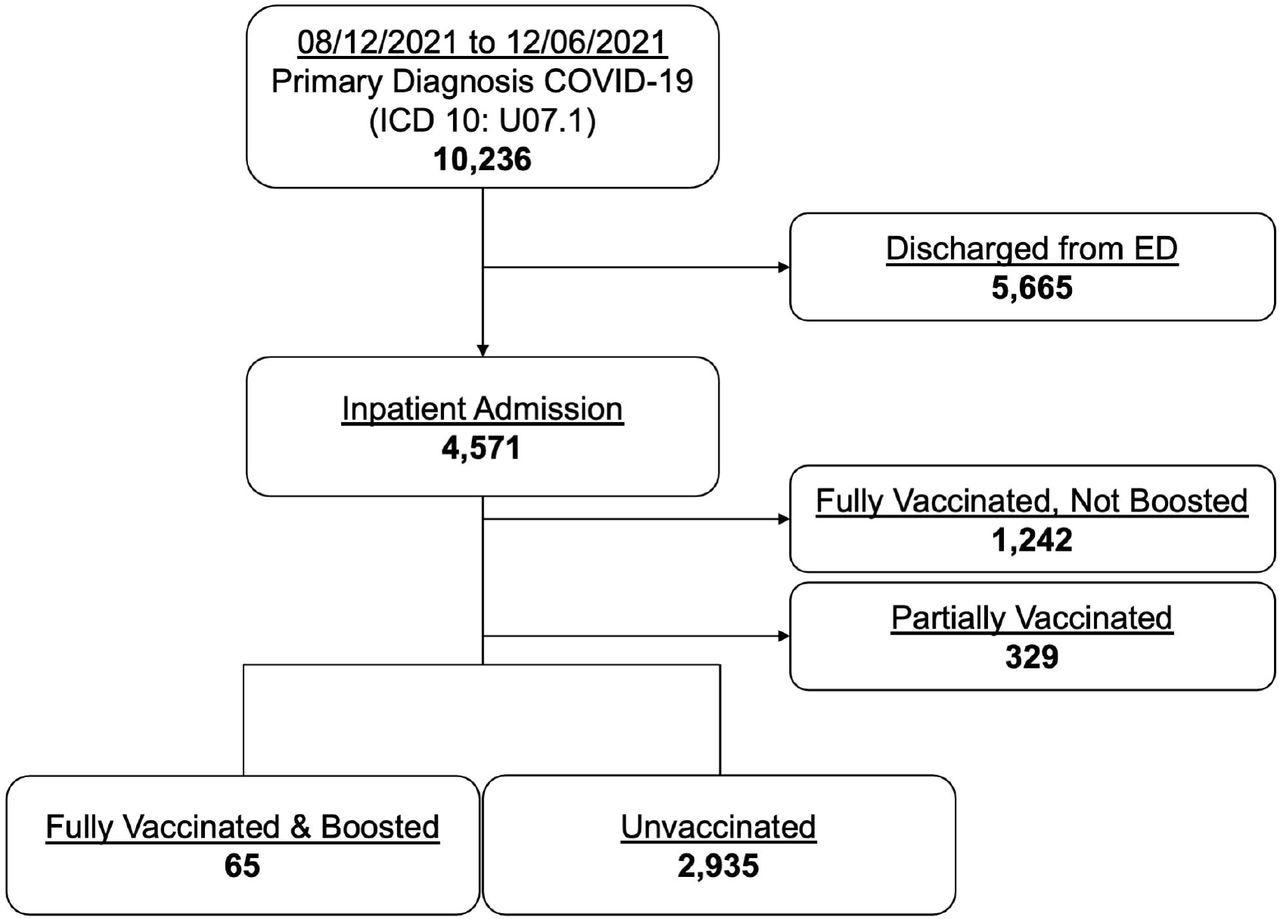
Enrollment profile of COVID-19 emergency and inpatient encounters.
Study findings
Between August 12, 2021, and December 6, 2021, 10,236 ED encounters with a primary diagnosis of COVID-19 were observed. Among these, encounters discharged from the ED, fully vaccinated but not boosted encounters, and partially vaccinated encounters were excluded from the study. Out of the remaining cases, 2.2% were FV&B and 97.8% were UV.
The median age of individuals in the FV&B cohort was 74 and that in the UV cohort was 58. In addition, the percentage of immunocompromised individuals and those with pre-existing end-stage renal disease (ESRD) in the FV&B cohort were 32.3% and 18.5%, respectively, while in UV individuals were 0.4% and 1.8%, respectively.
Mechanical ventilation was needed in 7.7% of FV&B patients and 11.1% of UV patients. In-hospital mortality was 7.7% and 12.1% for the FV&B and the UV groups, respectively.
Nearly 91% of the FV&B individuals exhibited upper respiratory symptoms or systemic symptoms, 84.6% had respiratory symptoms, 66.2% exhibited systemic symptoms, and 53.8% exhibited fatigue. COVID-19 symptoms were shown by 42% of the patients less than 12 days after receiving the booster dose. Among the patients requiring ICU care, 45% experienced COVID-19 symptoms less than 12 days after receiving the booster dose.
Almost 64% of the FV&B patients requiring ICU care were males of a median age of 71. Among FV&B patients who required ICU care, 36.4% died, 27.2% were discharged to a skilled nursing facility (SNF), 18.2% were discharged to a rehabilitation center, and 18.2% were discharged home.
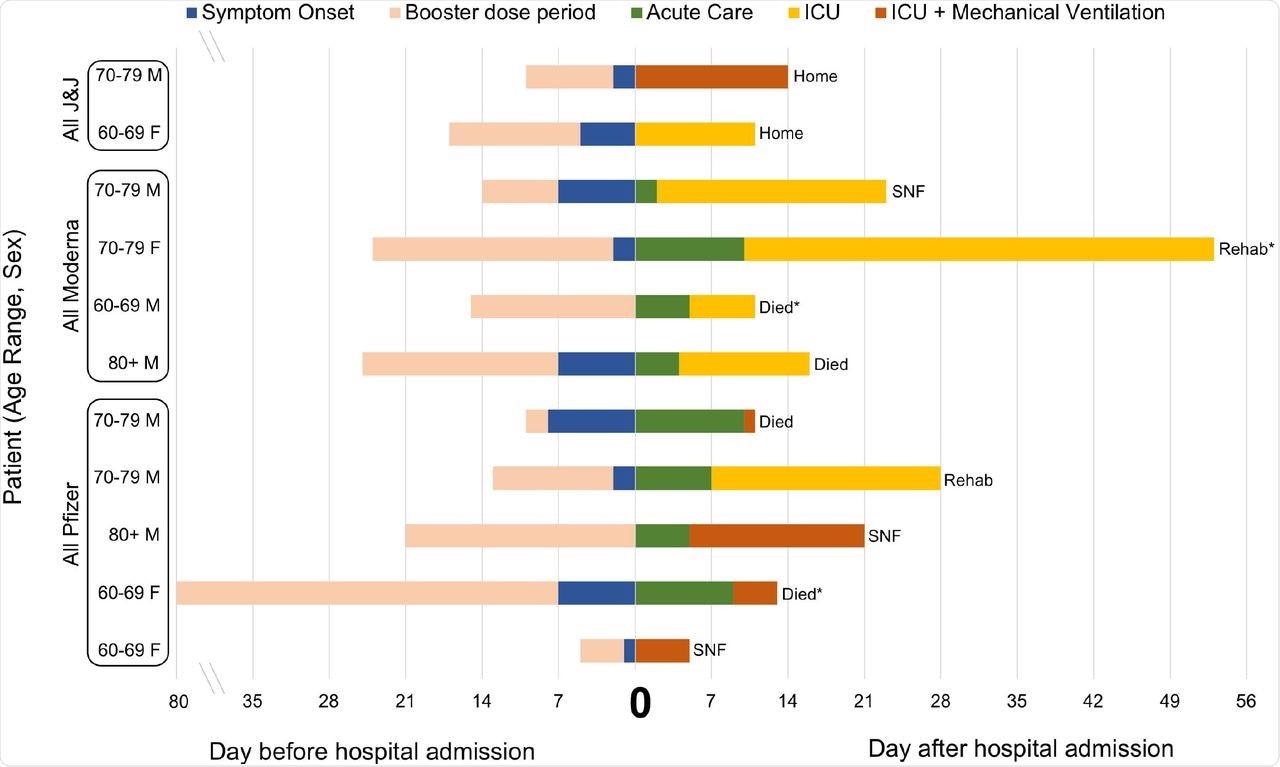
Characteristics and outcomes of 11 fully vaccinated and boosted patients with COVID-19 requiring intensive care admission.
Conclusion
The researchers concluded that booster doses provided protection against severe COVID-19 symptoms and also observed that the number of patients requiring mechanical ventilation and use of vasopressors, as well as in-hospital mortality, were lower in the FV&B cohorts as compared to the UV cohorts, despite a higher risk for in-hospital mortality in the FV&B cohort.
When the researchers compared the FV&B patients who required ICU care to those who did not, it was observed that there may not have been enough time for the booster dose in these patients to be effective in preventing COVID-19 symptoms.
Decreased in-hospital mortality was observed in FV&B patients compared to UV patients, even though the former group was older and had a higher rate of pre-existing ESRD, higher proportion of immunocompromised state, and a higher proportion of immunocompromised state risk of in-hospital death in the FV&B cohort. It was observed that even among elderly patients with significant comorbidities, a booster dose might safeguard the patient against increased severity of symptoms.
Taken together, further investigations are required to understand the protection provided by booster vaccines and diagnose the risk factors in the FV&B population.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources