 Study: Antibody and T cell responses to SARS-CoV-2 mRNA vaccines during maintenance therapy for immune-mediated inflammatory diseases. Image Credit: peterschreiber.media / Shutterstock
Study: Antibody and T cell responses to SARS-CoV-2 mRNA vaccines during maintenance therapy for immune-mediated inflammatory diseases. Image Credit: peterschreiber.media / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study details
From January 8 to October 4, 2021, the researchers invited patients with immune-mediated inflammatory diseases undergoing maintenance therapy to take part in the IMPACT study. The goal was to study antibody and T-cell response following a COVID-19 mRNA vaccine. The patients were immunized with either the two-dose Pfizer-BioNTech and/or Moderna vaccines.
There was a median of 60.5 days between the two doses. This was because patients recruited from Toronto, Canada, had a longer vaccine schedule than the standard 21 or 28 days in between doses.
All adult patients were undergoing anti-TNF treatment, anti-IL-17 therapy, methotrexate or azathioprine monotherapy or combination therapy with anti-TNF therapies, anti-IL-12/23 therapy, or anti-IL-23 therapy. One group of patients with immune-mediated inflammatory diseases was not taking any immunosuppressants.
A separate cohort of volunteers without immune-mediated inflammatory disease and not taking immunosuppressants served as the control group for comparison purposes.
Patients who received a COVID-19 vaccine other than mRNA, younger than 18, taking oral steroids, or had a previous COVID-19 infection were excluded from the study.
A total of 150 patients met the inclusion criteria for the study. The team collected blood and plasma samples at four time points: before vaccination, 2-4 weeks after the first dose, 2-4 weeks after the second dose, and three months after vaccination.
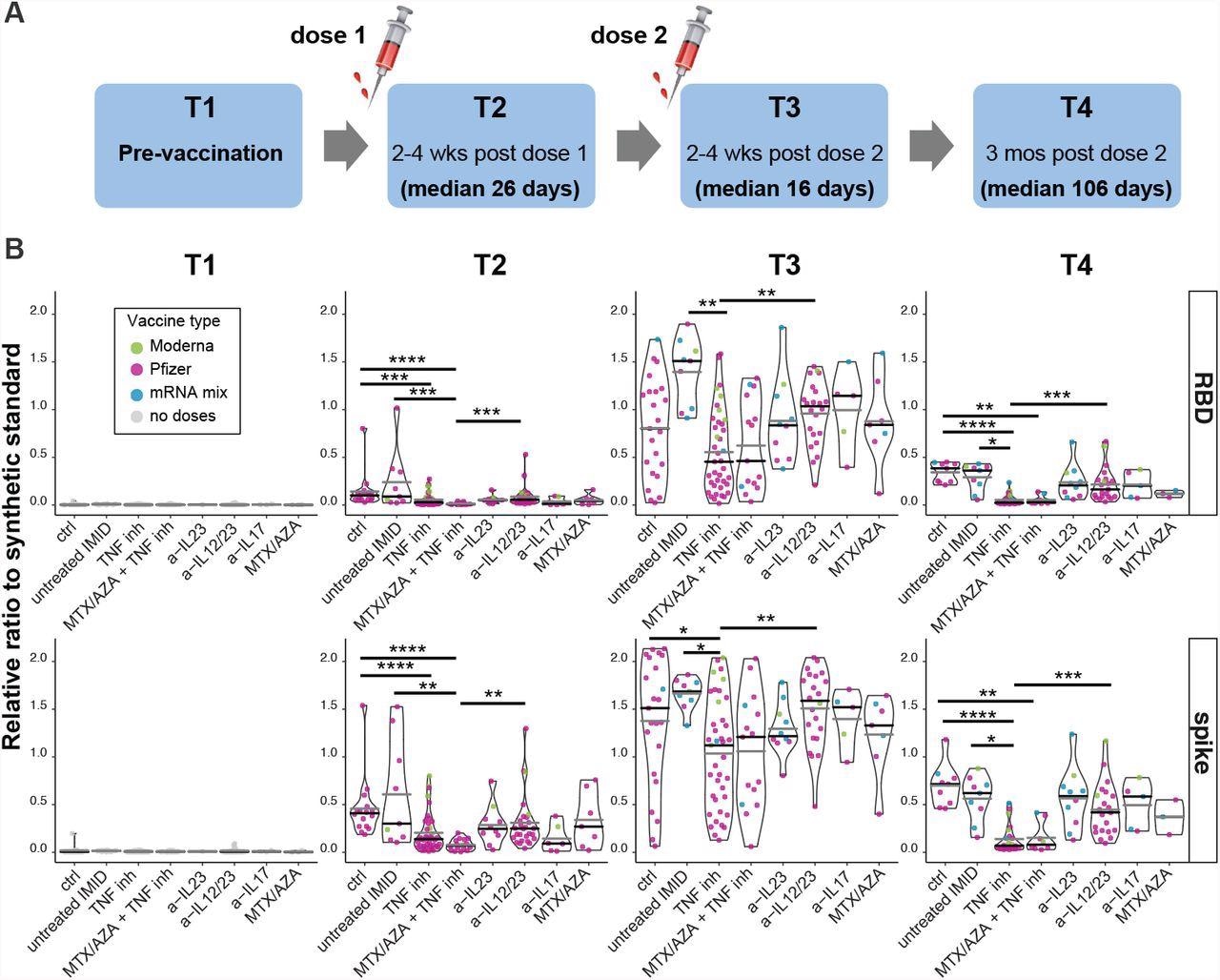
Antibody responses after 1 or 2 doses of mRNA vaccine. (A) Schematic diagram of the sampling schedule. (B) IgG responses before, and after the first and second doses of mRNA vaccine in IMID patients. Violin plots show the relative ratio of RBD and spike that were determined at the indicated time points in IMID patients under mono- and combination therapy (0.0039 µl sample used, see suppl. fig. 2B for the second dilution and suppl. table S1 for conversion to BAU/ml). T1, n=111; T2 n= 131, T3, n=131, T4, n= 88. The dot colors indicate the type of vaccine, Pfizer refers to BNT162b; Moderna to mRNA-1273; mRNA mix = first dose BNT162b, second dose mRNA-1273. MTX = methotrexate, AZA = azathioprine. Black and gray lines indicate median and mean ratio values for each violin, respectively. Plots are faceted based on the groups/treatments. Comparisons were made by Dunn’s multiple comparisons test based only on the BNT162b/BNT162b group. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001
Maintenance therapy and gender affected antibody responses
Antibody responses increased immediately after both vaccination doses.
After the first dose, about 97.8% of patients seroconverted to spike and 80% of patients seroconverted to RBD IgG antibodies.
After the second dose, there was a 100% seroconversion for SARS-CoV-2 spike protein and a 99.2% seroconversion for the receptor-binding domain.
The anti-spike and anti-RBD IgG levels from vaccinated patients were more significant than the levels of convalescent patients.
Two doses of the Moderna vaccine produced a greater immune response than two doses of Pfizer-BioNTech. Additionally, patients who mixed their mRNA vaccines had significantly higher levels of anti-spike/RBD IgG levels than two doses of the Pfizer-BioNTech vaccine.
Men had a lower antibody response targeting the receptor-binding domain than females. However, there was no difference in antibody response between ages.
Patients with an immune-mediated inflammatory disease undergoing immunosuppressive maintenance therapy had lower antibody responses than the control group.
Not all immunosuppressive therapies had the same reduction in antibody levels. Patients undergoing anti-TNF therapy and combination therapy with anti-TNF treatment had lower antibody levels than patients undergoing anti-IL-12/23 therapy after the first dose.
After the second dose, patients immunized with two doses of the Pfizer-BioNTech vaccine and undergoing anti-TNF therapy had lower IgG levels targeting the spike protein compared to the control group and patients taking anti-IL-12/23 therapy.
Reduced antibody response in immunosuppressive patients
The researchers noted that antibody levels waned three months after vaccination. The greatest reduction came from antibodies targeting the receptor-binding domain.
Only 66.3% and 49.4% of patients had more significant antibody responses for the spike protein and receptor binding domain than convalescent samples.
Patients undergoing anti-TNF therapy and combination therapy with anti-TNF treatment had a statistically significant reduction in IgG antibody levels than the control group and patients taking anti-IL-12/23 therapy.
Further, patients taking anti-TNF therapy had a lower neutralization response against the Beta, Delta, and Gamma variants than other groups.
T cell response after mRNA vaccination
One or two doses of an mRNA vaccine stimulated the production of cytokines IFN-γ, IL-2, IL-17A and IL-4 in all groups. The most significant boost came from IFN-γ and IL-2.
Although, after the first dose, there were deficits in IFN-γ production in all patients with immune-mediated inflammatory diseases, regardless of whether they were on maintenance therapy.
From the first to the second dose, there were significant increases in IL-2 levels. However, IL-4 levels decreased after 3 months.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Dayam RM, et al. (2022). Antibody and T cell responses to SARS-CoV-2 mRNA vaccines during maintenance therapy for immune-mediated inflammatory diseases. medRxiv. Doi: https://doi.org/10.1101/2022.01.26.22269856, https://www.medrxiv.org/content/10.1101/2022.01.26.22269856v1
- Peer reviewed and published scientific report.
Dayam, Roya M., Jaclyn C. Law, Rogier L. Goetgebuer, Gary Y. C. Chao, Kento T. Abe, Mitchell Sutton, Naomi Finkelstein, et al. 2022. “Accelerated Waning of Immunity to SARS-CoV-2 MRNA Vaccines in Patients with Immune-Mediated Inflammatory Diseases.” JCI Insight 7 (11). https://doi.org/10.1172/jci.insight.159721. https://insight.jci.org/articles/view/159721.