The current coronavirus disease 2019 (COVID-19) pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in a significant impact on global health. In most individuals, SARS-CoV-2 infection only causes mild disease; however, in a small minority of patients, COVID-19 can lead to severe respiratory failure and death.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Severe disease and increased risk of death associated with COVID-19 are observed in patients undergoing hemodialysis (HD). As a result, HD patients are among those prioritized to receive COVID-19 vaccinations.
Previous research has highlighted that HD patients elicit a diminished immune response after two doses of a COVID-19 vaccine. Although some research has tried to expand the understanding on this area, the T-cell interferon- γ (IFF-γ) response in HD patients following immunization has not been fully described. Due to the reduction of vaccine-induced antibodies and the rise of COVID-19 cases in Austria, the third dose of COVID-19 vaccines has been advised.
In a recent study published on the medRxiv* preprint server, researchers compare the impact of a third messenger ribonucleic acid (mRNA) COVID-19 vaccine in HD patients to healthy controls by measuring antibody levels and IFN-γ responses six to eight weeks after these individuals had received their third dose. Using this information, the authors were interested in establishing differences between the groups and determining whether further safeguards are required to appropriately protect this vulnerable population.
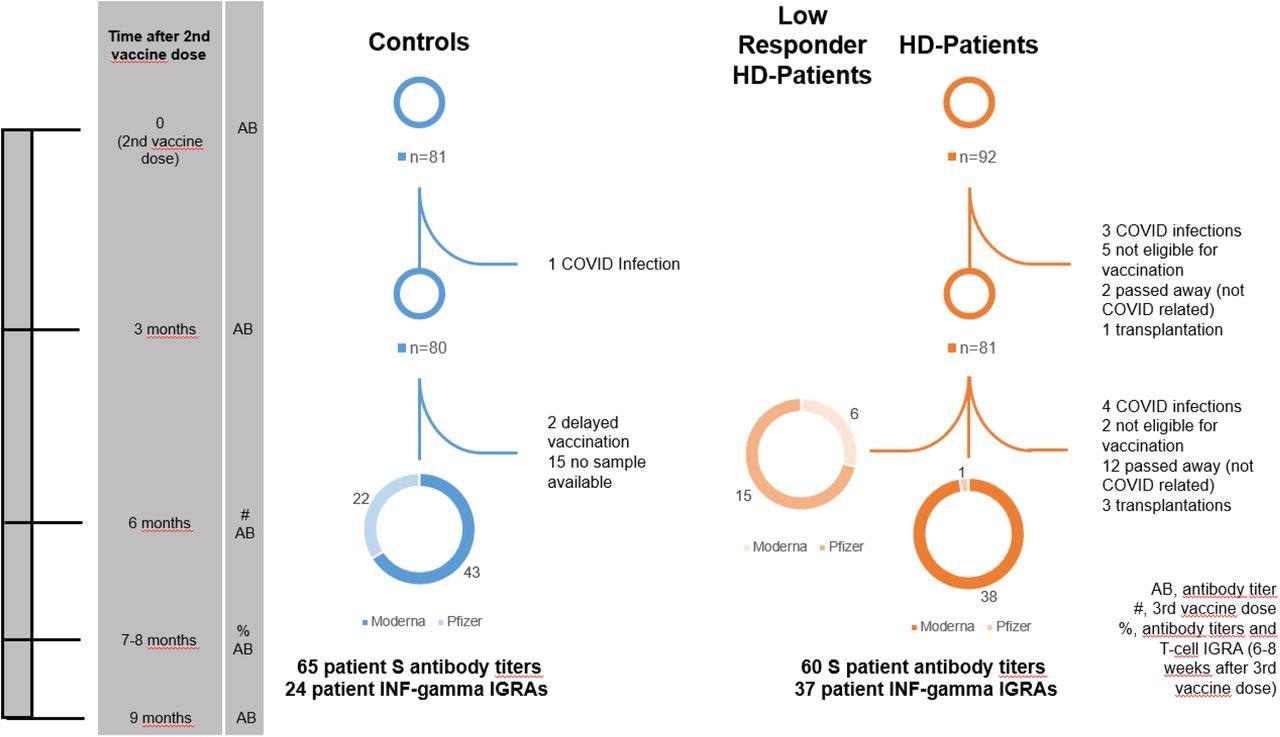
Patient flowchart. This flowchart is a graphical representation of the study design. The time axis on the left shows various significant time points in the study for easy orientation. The column beside the time points lists the events that occurred at this time point. Entries in this column are explained in the box at the bottom right of the flowchart.
About the study
The current prospective cohort study evaluated the antibody and IFN-γ response to two doses of the Pfizer/BioNTech BNT-162b2 mRNA vaccine followed by a booster mRNA vaccine dose. The eligibility criteria for HD patients included dialysis treatment for at least three months and receipt of a Comirnaty vaccine. The healthy control group consisted of volunteer healthcare workers who had received the same vaccinations as the HD patients.
Initially, 81 dialysis patients were invited to receive a third booster vaccine dose. Four of these individuals became infected with SARS-CoV-2, two were unable to be vaccinated due to high C-reactive protein (CRP) levels, three obtained a transplant, and 12 died. Finally, a total of 60 HD patients were included in the study to receive their third vaccine dose.
Comparatively, a total of 65 healthy people were included as controls in the current study.
Study findings
Antibody titers against the SARS-CoV-2 receptor-binding domain (RBD) in 65 healthcare workers and 60 HD patients were assessed. Six to eight weeks after receiving a third COVID-19 vaccine dose, 100% of the control group had neutralizing antibody titers exceeding 15 BAU/ml. After the third vaccine dose, 97% of HD patients seroconverted.
No significant difference was observed in the SARS-CoV-2 RBD-specific antibody titers when the control group was compared to the HD patient group six to eight weeks following the third dose. However, HD patients with low initial antibody titers or who were considered non-responders had considerably lower antibody titers after receiving their third vaccine dose than the control and HD patient responder groups.
IFN-γ titers that were greater than the cut-off level were observed in 96% of the healthcare workers and 76% of the HD patients. Thus, the distribution of IFN-γ secretion differs between the three groups. The only significant difference was observed in median IFN-γ quantile regression analysis of the control group as compared to previously low/non-responder HD patients.
Reports of adverse events (AE) between the two groups were evaluated and compared descriptively, with all data obtained through self-reported questionnaires. To this end, no participants in either of the groups reported AEs that required hospitalization or a visit to an emergency department. In comparison to HD patients, the control group reported more local and systemic AEs after receiving their third vaccine dose.
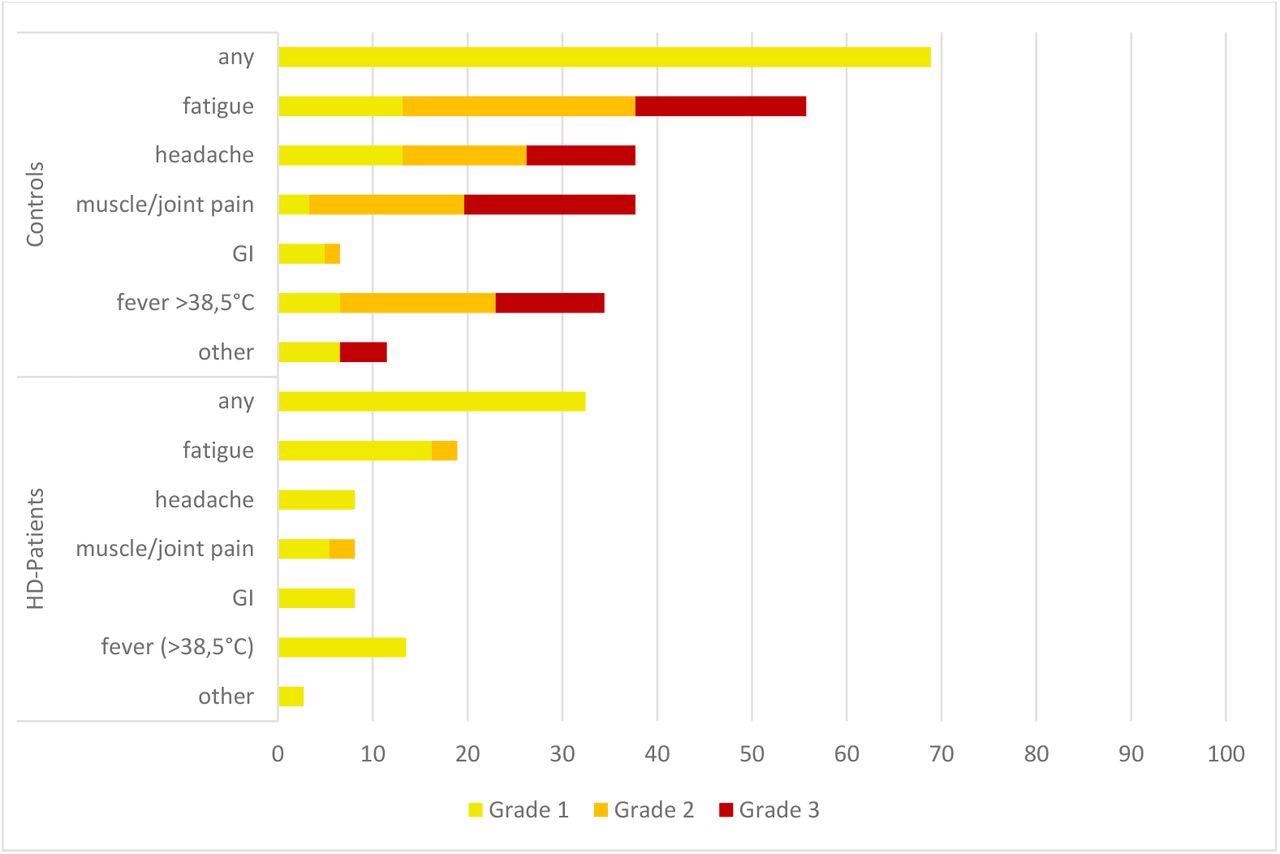
Systemic adverse events (AEs) after the third vaccination. All numbers represent the percentages of dialysis (n = 36) and control (n = 61) patients. The AEs were recorded using a standardized questionnaire and graded by the patients (Grade 1: mild, does not interfere with activity; Grade 2: moderate, interferes with activity; Grade 3: severe, prevents daily activity). No Grade 4 events (emergency department visits or hospitalization) were reported. HD patients, patients on hemodialysis; GI, gastrointestinal AEs (diarrhea, nausea and vomiting).
Implications
Taken together, the current study findings indicate that the third dose of a COVID-19 vaccine is a viable method for improving immune responses in most HD patients to levels comparable to healthy controls. To prevent HD patients from severe SARS-CoV-2 infection, more research on alternate immunization techniques should be conducted in the future.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Simon, B., Rubey, H., Gromann, M., et al. (2022). Severe Acute Respiratory Coronavirus-2 Antibody and T cell response after a third vaccine dose in hemodialysis patients compared with healthy controls. medRxiv. doi:10.1101/2022.03.16.22272527. https://www.medrxiv.org/content/10.1101/2022.03.16.22272527v1.
- Peer reviewed and published scientific report.
Simon, Benedikt, Harald Rubey, Martin Gromann, Astrid Knopf-Völkerer, Boris Hemedi, Sonja Zehetmayer, and Bernhard Kirsch. 2022. “SARS-CoV-2 Antibody and T Cell Response after a Third Vaccine Dose in Hemodialysis Patients Compared with Healthy Controls.” Vaccines 10 (5): 694. https://doi.org/10.3390/vaccines10050694. https://www.mdpi.com/2076-393X/10/5/694.