
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The SARS-CoV-2 Omicron BA.2 variant has fewer mutations in its spike protein than the Omicron BA.1 variant. However, BA.2 still shares some mutations with the BA.1 variant, in addition to several unique modifications.
The SARS-CoV-2 Omicron BA.4 and BA.5 sub-lineages emerged from the BA.2 lineage in 2022. Both BA.4 and BA.5 share an identical spike protein that is similar to that of BA.2, except for the 69-70 deletion, as well as L452R, F486V, and wildtype Q493 mutations. These mutations could potentially affect the action of therapeutic mAbs.
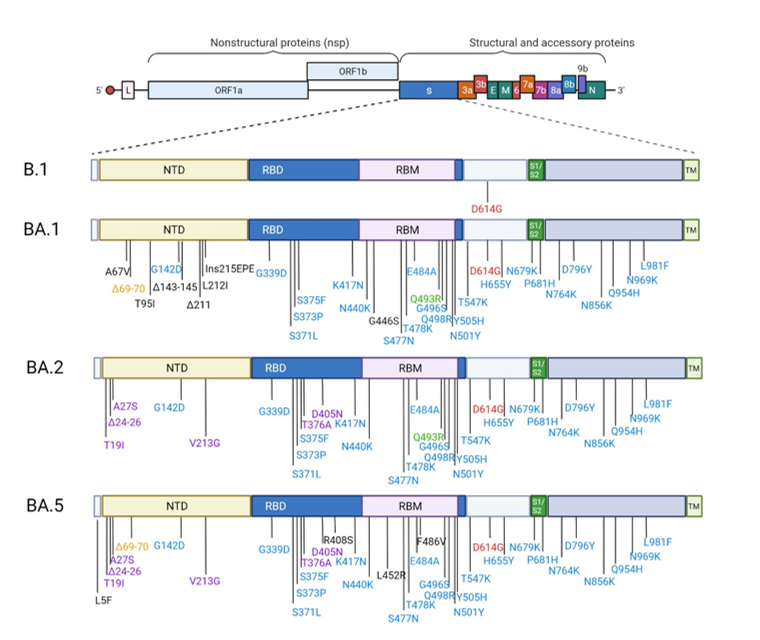
Spike substitutions in SARS-COV-2 variants Omicron BA.1, BA.2 and BA.5 compared to the ancestral strain B.1. Omicron BA.1, BA.2 and BA.5 sequences used for the representation are the one from the strains used in this study BA.1:EPI_ISL_7899754, BA.2: EPI_ISL_9426119 and BA.5: EPI_ISL_12852091.Red color indicates the mutation that is present in all strains. The blue color indicates the mutations which are common to BA.1, BA.2 and BA.5. The orange color indicates the mutations which are common to BA.1/BA.5. The purple color indicated the mutations which are common to BA.2/BA.5. This figure was created with BioRender.com.
About the study
In the present study, researchers evaluate the neutralizing activity of therapeutic mAbs against clinical strains of SARS-CoV-2 Omicron BA.1, BA.2, and BA.5 sub-lineages relative to the ancestral B.1 strain. Currently used therapeutic mAbs that neutralize the BA.1 variant were used, of which included sotrovimab (vir-7831), cilgavimab (AZD1061), tixagevimab (AZD8895), and Evusheld (AZD7442), the latter of which is a cocktail of tixagevimab and cilgavimab.
A standardized assay based on the reduction of ribonucleic acid (RNA) yield was performed in recombinant Vero E6 cells expressing the human transmembrane protease serine 2 (TMPRSS2). Two-fold serially diluted antibodies were added to the cells and inoculated with a 50% tissue culture infectious dose (TCID50) of SARS-CoV-2.
Viral RNA was quantified by reverse-transcription quantitative polymerase chain reaction (RT-qPCR) 48 hours after infection to estimate the half-maximal effective concentration (EC50). The researchers also calculated 50% millions of neutralizing units (MNU50), which is defined as the neutralizing capacity per treatment. One neutralization unit 50 (NU50) was the quantity of antibodies required for 50% neutralization of 100 TCID50 SARS-CoV-2.
Study findings
The neutralizing activity of tixagevimab was low against the Omicron sub-variants.
Comparatively, cilgavimab retained neutralization against BA.2 and BA.5, with a 2.6-fold and 1.2-fold reduction, respectively, relative to B.1. In contrast, the neutralizing activity against BA.1 was 84.2-fold lower than that against B1. Thus, cilgavimab retained a 32-fold higher neutralization against BA.2 than BA.1.
Evusheld exhibited a 1.9-fold loss of neutralization against BA.2 relative to B1, but a 15-fold increase in neutralization as compared to BA.1. A marginal decrease in neutralization was evident for the mAb cocktail against BA.5 as compared to BA.2. However, when compared to BA.1, this treatment exhibited a 10-fold increase in neutralization.
Sotrovimab treatment resulted in a 9.6-fold reduction in neutralization against BA.2 as compared to B1. Neutralization was also reduced by 18.7-fold against BA.5 as compared to B.1, but 2.7-fold relative to BA.1 and 1.9-fold compared to BA.2.
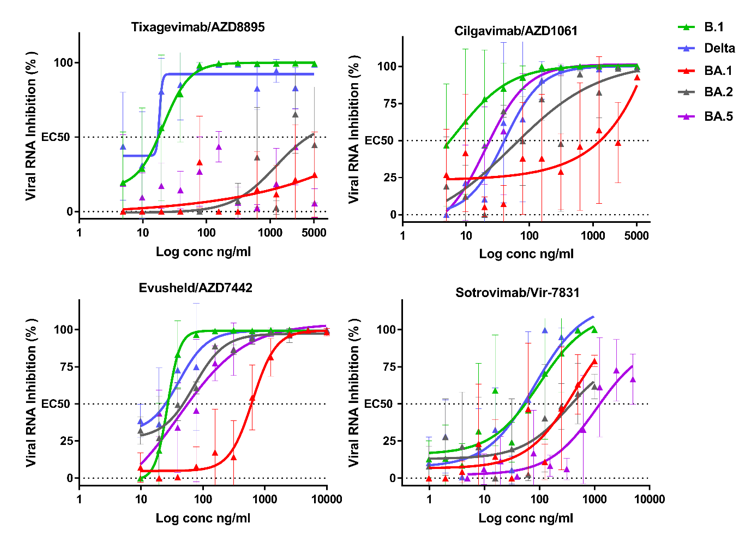
Dose response curves reporting the susceptibility of the SARS-CoV-2 BavPat1 D614G (B.1) ancestral strain,Delta BA.1 BA.2 and BA.5 variant to active therapeutic monoclonal antibodies Sotrovimab/Vir-7831, ,Tixagevimab/AZD8895, Cilgavimab/AZD1061 and Evusheld/AZD7742. Data presented are from one representative experiment. Data presented are from three technical replicates in VeroE6-TMPRSS2 cells, and error bars show mean±s.d.
Conclusions
Sotrovimab retained partial neutralization against BA.2 and BA.5. Furthermore, the neutralization activity of cilgavimab against BA.2 significantly improved the neutralization capacity per treatment of Evusheld (53.5 MNU50) against this variant.
The neutralizing activity of tixagevimab was not replicated against BA.2 and BA.5; therefore, future studies should assess whether its use in the cocktail is still relevant.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Touret, F., Baronti, C., Pastorino, B., et al. (2022). In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5 In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1 and BA.2. Research Square. doi:10.21203/rs.3.rs-1415749/v2. https://www.researchsquare.com/article/rs-1415749/v2
- Peer reviewed and published scientific report.
Touret, Franck, Cécile Baronti, Boris Pastorino, Paola Mariela Saba Villarroel, Laetitia Ninove, Antoine Nougairède, and Xavier de Lamballerie. 2022. “In Vitro Activity of Therapeutic Antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5.” Scientific Reports 12 (1). https://doi.org/10.1038/s41598-022-16964-z, https://www.nature.com/articles/s41598-022-16964-z