The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the coronavirus disease 2019 (COVID-19) pandemic. Since the beginning of the pandemic, several SARS-CoV-2 variants have emerged with different rates of transmission, virulence, and capacity to escape immune responses elicited through either COVID-19 vaccination or natural infection as compared to the ancestral variant.

 *Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
The SARS-CoV-2 Delta variant was the dominant circulating strain in Thailand by mid-August 2021. The spread of this variant significantly increased the number of COVID-19 cases and deaths.
During this period, the number of daily cases reached around 20,000, with over 300 deaths reported each day. The subsequent decline in COVID-19 cases occurred following the implementation of pharmaceutical and non-pharmaceutical measures.
Previous studies have indicated that COVID-19 vaccination has an important role in reducing daily infections and hospitalizations due to severe infection. According to the United States Centers of Disease Control and Prevention (CDC), COVID-19 vaccination, particularly when messenger ribonucleic acid (mRNA)-based vaccines are used, has significantly reduced the mortality rate of COVID-19.
Most of the population in Thailand has been vaccinated with inactivated vaccines, with a small percentage having received an mRNA vaccine as a booster vaccine dose.
The emergence of the SARS-CoV-2 Omicron variant in Thailand caused a surge in daily infections that reached almost 50,000 per day. However, the number of deaths due to SARS-CoV-2 infection remained low, at about 120 each day.
Scientists have previously hypothesized that herd immunity would be developed after a majority of the population was vaccinated or recovered from COVID-19. However, the herd immunity threshold could not be reached due to the emergence of new variants, as well as vaccine hesitancy.
A United Kingdom study coined the concept of "hybrid immunity," which refers to the protection conferred by COVID-19 vaccination and natural infection. Previous studies have indicated that hybrid immunity can protect individuals from symptomatic infection.
About the study
In a new study under review in the Scientific Reports journal and currently available on the Research Square* preprint server, scientists hypothesize that lower death rates in COVID-19 patients may be due to hybrid immunity, as well as the reduced severity of Omicron infections.
The current study included 79 participants from 15 families registered in a database of the Bangkok home healthcare service between August 1, 2021, and August 31, 2021. In this study cohort, 34 individuals had recovered from COVID-19 at least four weeks before enrollment, whereas the remaining 45 participants were in close contact with COVID-19 patients.
Study findings
T-cell responses against Neuromyelitis optica (NMO) antigens were detected through the interferon release assay in 11 out of 45 close contacts six months following SARS-CoV-2 exposure. The rate of asymptomatic COVID-19 was estimated to be 24.4%.
The SARS-CoV-2 receptor-binding domain (RBD) immunoglobulin G (IgG) antibody levels, as well as T-cell responses against the SARS-CoV-2 spike protein, following the second COVID-19 vaccine dose was associated with comparable immunity between COVID-19 patients and close contacts. Thus, participants who received COVID-19 booster vaccination without any history of prior COVID-19 also benefited from hybrid immunity.
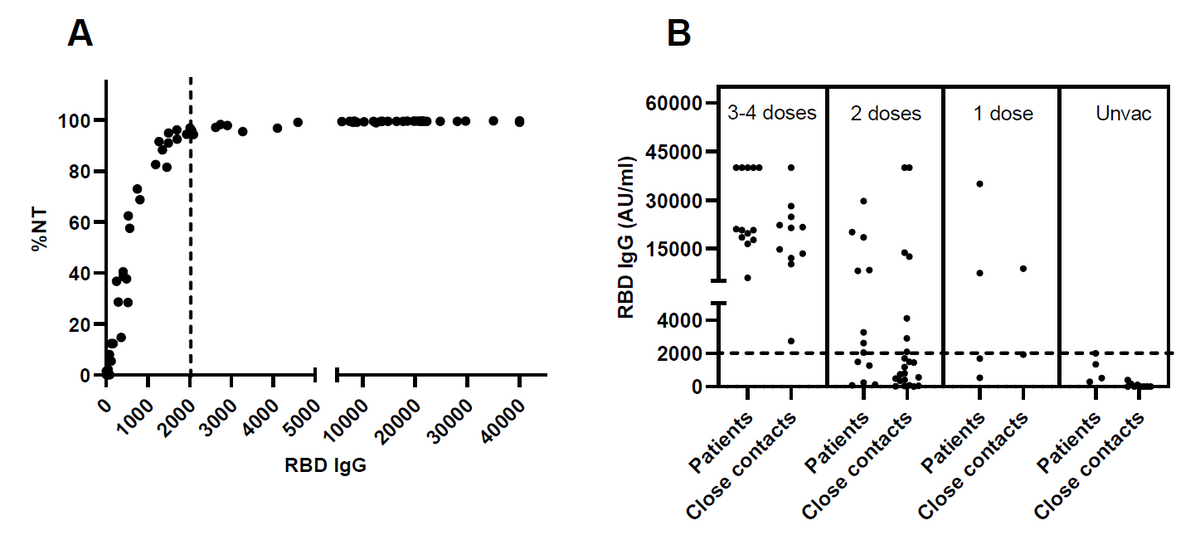
The antibody response to RBD IgG (Figure 2A) demonstrated the correlation between the levels of antibody and neutralization capacity to the alpha variant of SAR-CoV-2 virus (R = 0.5571, P < 0.0001). The levels of RBD IgG in COVID-19 patients and closed contacts according to the number of vaccinations are presented in Figure 2B. NT = neutralizing antibody, RBD IgG = SARS-CoV-2 receptor binding domain immunoglobulin G, AU/ml = arbitrary units per milliliter.
A similar level of immunity was observed among individuals who were in close contact with asymptomatic and symptomatic infected persons.
Previously, long-term observational data was not available related to the reinfection rate in individuals who were exposed to SARS-CoV-2. In the present study, none of the participants were reinfected with SARS-CoV-2 during enrollment.Figure 3.
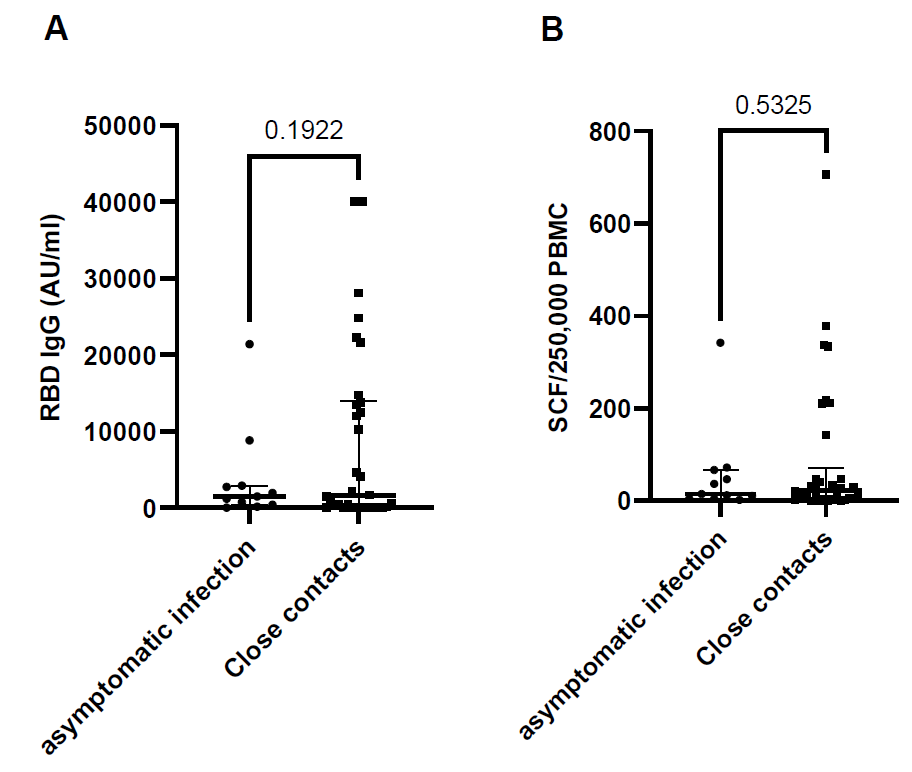
Immune response against SARS-CoV-2 viral antigens compared between close contacts with and without asymptomatic infection, antibody response (RBD IgG) P = 0.1922 t=1.325, df=43 (Figure 3A), T cell response against spike protein P = 0.5325, t=0.6293, df=43 (Figure 3B). RBD IgG = SARS-CoV-2 receptor binding domain immunoglobulin G, AU/ml = arbitrary units per milliliter.
Most of the study participants had received heterogenous COVID-19 booster vaccination that included viral vector vaccines, inactivated vaccines, and mRNA vaccines. To this end, reduced T-cell responses against the spike protein and higher RBD IgG levels were observed post-booster vaccination.
After a short period of depletion, T-cells may recover three months after receipt of a COVID-19 booster dose. This observation aligns with a previous study that reported a retained effective immune response following heterogeneous immunization against the Omicron variant.
Previous studies have revealed that individuals may possess varied levels of cellular and humoral immune responses during similar viral infections. Since the majority of the study participants received an mRNA vaccine as the booster dose, it might alter T-cell function after vaccination.
Thus, the authors strongly recommend multiple booster doses of the COVID-19 vaccine only for immunocompromised individuals exhibiting poor T-cell responses. T-cell-based vaccines would also provide an enhanced therapeutic benefit, particularly for these patients.
Conclusions
The current study revealed that both antibody and cellular responses define COVID-19 immunity in society. Taken together, these responses may confer long-term hybrid immunity following booster vaccination against symptomatic and asymptomatic COVID-19.
An important limitation of the current study is its small size, which limits the generalizability of its results. Furthermore, the immune response was confounded by vaccination.

 *Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.