AN, a disorder associated with altered eating, has caused considerable mortality, especially among women. However, therapies based on scientific evidence are scarce. AN pathogenesis likely involves several environmental and genetic factors. Studies have reported intestinal microbial dysbiosis among AN-affected individuals. However, data were obtained from small sample sizes, and genus-level microbial alterations were analyzed by amplicon sequencing.
 Study: The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Image Credit: Tatiana Shepeleva / Shutterstock
Study: The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Image Credit: Tatiana Shepeleva / Shutterstock
About the study
In the present study, researchers assessed the association between the intestinal microbiome and AN.
The team performed metabolomics and shotgun metagenomic analyses on serum and fecal samples, respectively, that were obtained from women with AN (n=77) and age- and sex-matched healthy controls (n=70). Further, the fecal microbiome was transplanted from anorexia nervosa cases to murine animals fed calories-limited diets over three weeks to simulate AN eating behavior for in vivo analysis. In addition, the team explored causal associations in silico by bidirectional mediation analysis. The intestinal microbiome was analyzed at functional, taxonomic, and genetic levels.
The team used the eating disorder inventory-3 (EDI-3) questionnaire to assess eating behaviors and insulin resistance was assessed using the homoeostatic model assessment for insulin resistance (HOMA-IR) tool. The team examined covariations between bacterial abundance at species and genus levels and clinical variables for AN cases and controls. Linear regression modeling was performed, adjusting for confounders such as age, smoking status, medications, and body mass index (BMI).
Further, the team evaluated the growth dynamics of gut bacteria by calculating peak-to-trough ratios (PTR) using the metagenomic dataset. The functional modules of gut bacteria were identified using gut-brain modules (GBMs) and gut metabolic modules (GMMs). Differences in bacterial genomics were explored based on the Canberra distance of bacterial structural variant profiles.
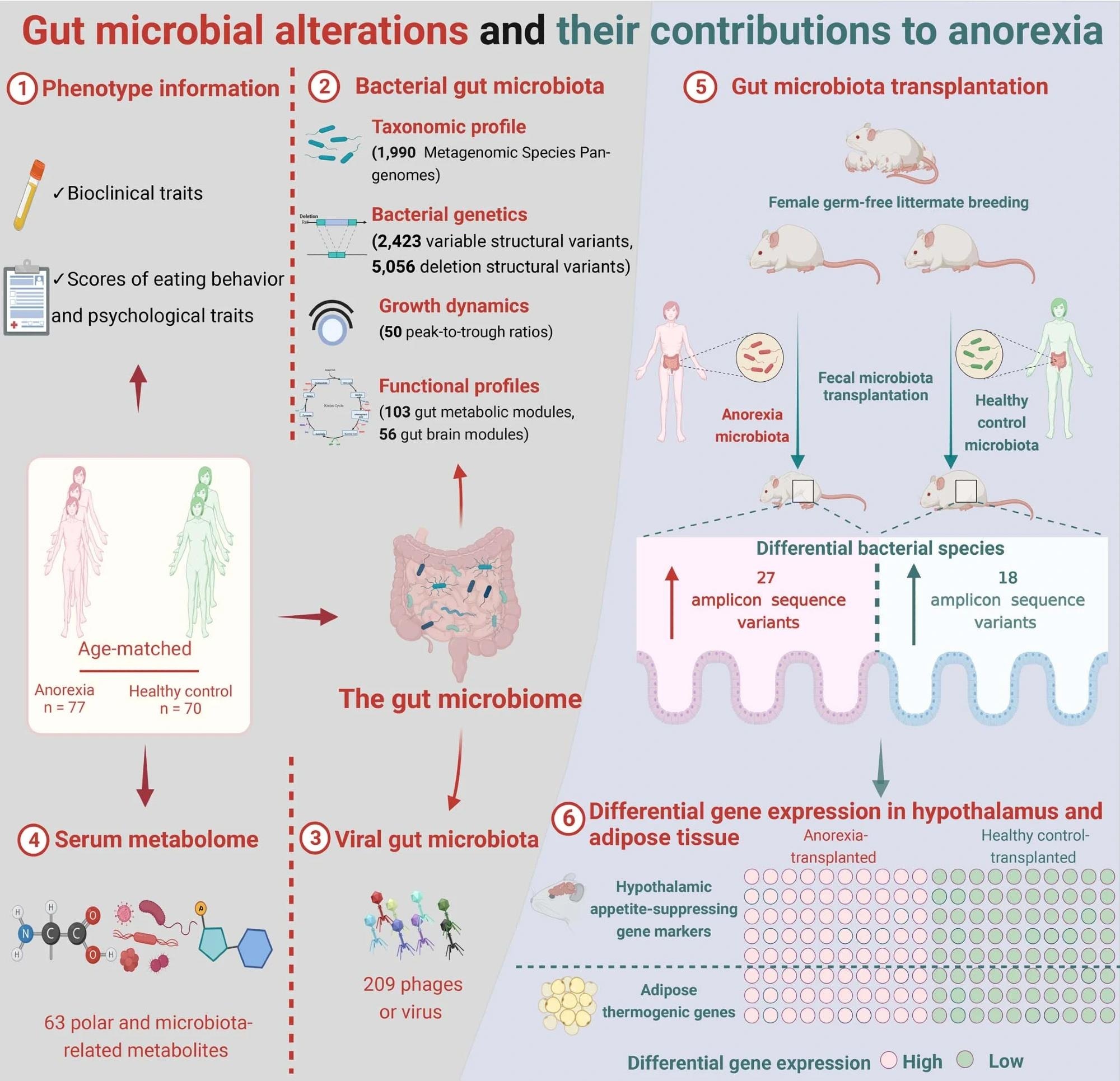 Graphical abstract of the study workflow and findings.
Graphical abstract of the study workflow and findings.
Results
Several bacterial organisms (including Clostridium) were altered among individuals with anorexia nervosa and were associated with mental well-being and eating behavior estimates. Bacterial functional-type modules related to neurotransmitter degradation were enriched among those with anorexia nervosa. Further, several structural variants (SVs) in bacterial organisms were associated with the metabolic characteristics of anorexia nervosa.
The findings indicated a probable role of the intestinal microbiome in AN-associated changes concerning satiety and the metabolism of secondary bile acids. The metabolomic analysis indicated an elevation in metabolites linked to lowered food consumption (including taurine-hyodeoxycholic acid, taurine-α-muricholic acid, and indole-3-propionic acid molecules). Causal inference analysis indicated that serological bacterial metabolites probably mediate the effect of gut microbial alterations on anorexia nervosa. At the phylum level, AN microbiome samples showed lowered Actinobacteriota and Bacteroidota counts. Among families of bacteria, Christensenellaceae species, particularly CAG-138, showed the most significant enrichment in AN.
At the genus level, elevated Lactobacillus counts were observed in the AN microbiota. The Ruminococcacea-enterotype was more prevalent in cases of AN. Species-level analysis indicated greater β-diversity among AN-affected women. In AN, Roseburia inulinivorans and Roseburia intestinalis were depleted, whereas those of Erysipelatoclostridium ramosum, Blautia species CAG, and Enterocloster bolteae innocuum (Clostridium) were increased. Clostridium counts correlated positively with eating disorder scores. The abundance of Bifidobacterium and Parasutterella, in absolute terms, showed positive correlations with perfectionism and body dissatisfaction, respectively.
Absolute Brachyspira count showed a positive association with ‘drive for thinness’ markers in anorexia nervosa. Median values for PTR markedly differed between individuals with AN and controls. Women with AN were leaner, had lower fasting serological insulin, glucose, and C-reactive protein (CRP) levels, and were more sensitive to insulin than controls. Bacterial organisms with significant growth retardation, among AN case individuals included Alistipes finegoldii, Akkermansia muciniphila, Eubacterium siraeum, Coprococcus catus, SS3/4, and Odoribacter splanchnicus.
In addition, the intestinal virome was altered among AN-affected individuals, including lowered bacterial-viral interactions, due to attenuated interactions of viruses with short-chain fatty acid (SCFA)-producing bacteria, including Roseburia inulinivorans, Roseburia hominis, and Faecalibacterium prausnitzii. The team observed greater viral richness and Shannon diversity in the fecal samples of AN cases compared to controls. Notably, 25/30 viruses increased in AN were Lactococcus bacteriophages. The abundance of GBMs for serotonin synthesis and degradation of tryptophan, glutamate, and dopamine, were enriched in AN.
The team detected 2,423 and 5,056 variable SVs and deletion SVs, respectively, across 56 species of bacteria, including Bacteroides uniformis, Faecalibacterium prausnitzii, Parabacteroides distasonis, Methanobrevibacter smithii. Individuals with AN lacking the genomic region of B. uniformis had greater scores for self-denial and bulimia. The genetic deletion in B. uniformis could result in the deficiency of thiamine, a vitamin associated with intestinal and mental health. The serotonin synthesis module causally affected BMI through glycoursodeoxycholic acid, which is upregulated by serotonin.
Serum leucine mediated the influence of B. vulgatus counts on glucose homeostasis. Mice receiving AN individuals’ fecal transplants initially lost more weight with a slower gain of weight with time than those receiving fecal transplants of control individuals. The finding was related to greater levels of hypothalamic appetite-suppressing genes and thermogenesis-associated genes in the adipose tissues of mice receiving fecal transplants from individuals with AN.
Based on the study findings, gut microbial disruptions may contribute to the pathogenesis of AN.