In a recent study published in the Proceedings of the National Academy of Sciences, researchers explored the shortcomings in mucosal immunoglobulin A (IgA) responses induced by existing vaccines against the Omicron variant of severe acute respiratory disease coronavirus 2 (SARS-CoV-2). To address this, they engineered IgA antibodies from four immunoglobulin G (IgG) monoclonal antibodies (mAbs) capable of neutralizing the Omicron variant. They found that the derived IgA antibodies improved neutralization by up to 75-fold and conferred protection against Omicron BA.5 in mice models.
 Study: Conversion of monoclonal IgG to dimeric and secretory IgA restores neutralizing ability and prevents infection of Omicron lineages. Image Credit: Christoph Burgstedt / Shutterstock
Study: Conversion of monoclonal IgG to dimeric and secretory IgA restores neutralizing ability and prevents infection of Omicron lineages. Image Credit: Christoph Burgstedt / Shutterstock
Background
The ongoing evolution of SARS-CoV-2 has led to the emergence of variants of concern (VOCs). The Omicron variant has particularly raised concerns due to its numerous spike protein mutations, especially considering widespread reinfections and breakthrough infections (BTIs). As Omicron is characterized by multiple lineages and sub-variants, it poses challenges to existing antibody treatments, highlighting the need for new and improved antibody therapies. Considering the shift in Omicron's tropism towards the upper respiratory tract, leveraging mucosal immunity becomes crucial for therapeutic or prophylactic interventions. Therefore, researchers in the present study engineered recombinant monomeric (mIgA), dimeric (dIgA), and secretory immunoglobulin A (sIgA, the predominant Ig type in mucosal defenses) from neutralizing IgG antibodies. The study aimed to investigate the potential of delivering both dIgA and sIgA through a nasal spray to enhance mucosal immunity and counter SARS-CoV-2 infections effectively.
About the study
In the present study, researchers assessed the salivary antibody levels targeting the receptor-binding domains (RBDs) of both the G614 (wild-type) and all Omicron lineages. This evaluation involved vaccinated individuals (n = 2) who had received different doses of inactivated vaccines, heterologous vaccines (combining inactivated vaccines with mRNA (short for messenger ribonucleic acid) vaccines), or those with confirmed BTIs characterized by mild symptoms (n = 13).
Further, they aimed to develop an effective IgA mAb therapy that can be administered directly at the mucosal surface. Four neutralizing IgG mAbs (01A05, rmAb23, DXP-604, and XG014) targeting the RBD were identified for conversion into mIgA1, dIgA1, and sIgA1 forms. Their distinct binding preferences and cross-neutralizing capacities were characterized using computational structure modeling and biochemical assays. The effective concentrations (EC50) and inhibitory concentrations (IC50) of IgA1 antibodies were measured and compared. Transgenic mice (n = 3) expressing human angiotensin-converting enzyme 2 (ACE2) were used to visualize the biodistribution and test the prophylactic and therapeutic protection offered by the IgA1 antibodies against Omicron BA.5. Additionally, pseudo-virus assays were used to assess the neutralization activity of DXP-604 IgG and IgA1 against circulating Omicron sub-variants.
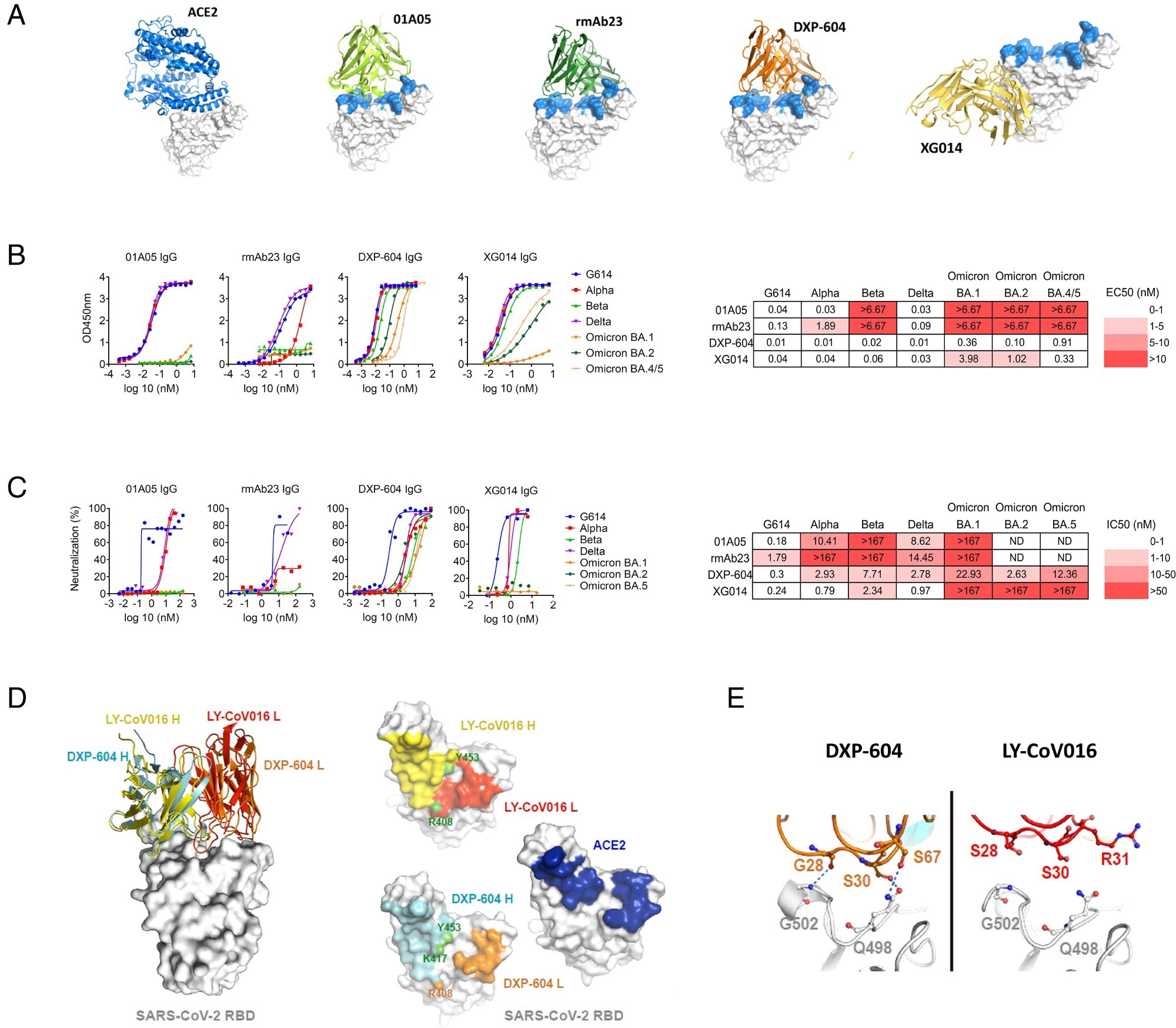 Characterization of neutralizing antibodies 01A05, rmAb23, DXP-604, and XG014. (A) In silico binding of ACE2 and IgG antibodies to RBD. The ACE2 receptor binding motif is indicated (blue). (B and C) Binding to RBD (B) and neutralization (C) of G614 and VOCs by IgG antibodies. The EC50 and IC50 and fold-change differences between IgG and IgA antibody forms are indicated. (D) Overlaid crystal structures of LY-CoV016 Fab (PDB ID: 7C01) and DXP-604 Fab 473 (PDB ID: 7CH4) in complex with SARS-CoV-2 RBD (Left picture) and the footprints of LY-CoV016, DXP-604, and ACE2 (PDB ID: 6M0J) on SARS-CoV-2 RBD. Atoms of the RBD within 5.0 Å of the antibodies or ACE2 are colored yellow (LY-CoV016 H), red (LY-CoV016 L), cyan (DXP-604 H), orange (DXP-604 L), or blue (ACE2) (Right picture). (E) Hydrogen bonds were formed between S30/S67 in the light chain of DXP-604 and RBD Q498, which is a key ACE2-binding site, and between the main chain groups of G28 and RBD G502.
Characterization of neutralizing antibodies 01A05, rmAb23, DXP-604, and XG014. (A) In silico binding of ACE2 and IgG antibodies to RBD. The ACE2 receptor binding motif is indicated (blue). (B and C) Binding to RBD (B) and neutralization (C) of G614 and VOCs by IgG antibodies. The EC50 and IC50 and fold-change differences between IgG and IgA antibody forms are indicated. (D) Overlaid crystal structures of LY-CoV016 Fab (PDB ID: 7C01) and DXP-604 Fab 473 (PDB ID: 7CH4) in complex with SARS-CoV-2 RBD (Left picture) and the footprints of LY-CoV016, DXP-604, and ACE2 (PDB ID: 6M0J) on SARS-CoV-2 RBD. Atoms of the RBD within 5.0 Å of the antibodies or ACE2 are colored yellow (LY-CoV016 H), red (LY-CoV016 L), cyan (DXP-604 H), orange (DXP-604 L), or blue (ACE2) (Right picture). (E) Hydrogen bonds were formed between S30/S67 in the light chain of DXP-604 and RBD Q498, which is a key ACE2-binding site, and between the main chain groups of G28 and RBD G502.
Results and discussion
Results revealed lower salivary IgA and IgG levels against Omicron variants in vaccinated individuals, while BTI participants displayed a sustained and broadly cross-reactive mucosal immune response with a significant increase in salivary antibodies. Salivary anti-RBD IgA levels correlated strongly with RBD-specific secretory Ig, emphasizing their local production, while salivary IgG levels showed a correlation with plasma IgG antibodies, suggesting passive diffusion. The study highlights the importance of measuring mucosal immunity to comprehensively understand immune responses to SARS-CoV-2 variants.
Among the four IgG antibodies, DXP-604 was found to tolerate RBD substitutions better and showed a broad neutralization activity. Conversion of parental IgG to dIgA1 and sIgA1 was found to significantly increase the binding and neutralizing potency (25–75-fold) of the antibodies against various VOCs. The increased neutralizing potency of dIgA1 and sIgA1 is partly attributed to increased avidity via inter-S-trimer binding on the viral surface, with potential involvement of other mechanisms like inter-virion aggregation.
In mice models, intranasally administered DXP-604 dIgA1 was found to majorly target the respiratory tract and persist in the lungs for at least 48 h. Intranasal administration of DXP-604 dIgA1 showed a significant reduction in the mean viral load, indicating the strong therapeutic and prophylactic potential of the antibody. dIgA1 and sIgA1 were observed to significantly enhance the neutralization of BA.1, BA.2, and BA.4/5 Omicron subvariant pseudo-viruses by 35.4- to 110-fold compared to monomeric IgG.
Although the study is limited by its small sample size, the approach to engineer and intranasally deliver IgA1 shows promise in improving post-exposure prophylaxis in high-risk settings, where the intervention window is relatively small. Further research needs to be conducted in humans to confirm these findings.
Conclusion
In conclusion, engineering and delivering dimeric or secretory forms of monoclonal IgA antibodies through nasal spray together form a potent strategy to restore and enhance neutralizing ability, providing prophylactic and therapeutic protection against the Omicron variant. The findings suggest that in the future, a cocktail of dIgA1 and sIgA1 antibodies, including DXP-604, could help neutralize most of the emerging Omicron sub-variants and VOCs.