A new review shows how maternal microbes, milk molecules, and modern lifestyles interact to shape babies’ gut health, pointing to opportunities for prevention that span families and generations.

Review: Microbiome in heritage: how maternal microbiome transmission impacts next generation health. Image Credit: Gorodenkoff / Shutterstock
In a recent review published in the journal Microbiome, researchers synthesized evidence on how maternal intestinal bacteria, human milk components, and the maternal environment influence infant microbiome development and downstream risks for non-communicable diseases, highlighting preventive opportunities and research gaps.
Background
Within hours of birth, tiny microbes begin to settle in a baby’s gut and grow quickly over the first years. What parents eat, how the baby is born, how the baby is fed, and which microbes and helpful molecules come from the mother all shape this first community. Because much of the human evidence is associative, animal studies have played a key role in clarifying causality. These early choices can teach the immune system to live peacefully with friendly microbes or can raise the risk of allergies, obesity, type 2 diabetes mellitus, and inflammatory bowel disease later in life. Modern living has lowered infections, but many non-communicable diseases have increased. Further research is needed to translate this science into a practical and equitable prevention for all families.
Why the First Microbes Matter
Early life is a highly plastic period during which the infant's intestinal environment and microbiome develop in tandem. This crosstalk teaches the immune system what to tolerate and what to fight. When development proceeds smoothly, immune tolerance is fostered; when it is disrupted, susceptibility to non-communicable diseases increases. These dynamics position early microbial exposures as a powerful lever for long-term health. The review also notes that early microbiome disruptions may affect male and female immune and metabolic outcomes differently.
Modern Lifestyles, New Pressures
Industrialization transformed diet, hygiene, and medical care. While these advances reduced infectious diseases, they coincided with rising autoimmune diseases such as type 1 diabetes mellitus and multiple sclerosis, and with chronic inflammatory conditions including obesity, type 2 diabetes mellitus, and inflammatory bowel disease. Growing evidence suggests that early-life disturbances in host-microbiome interactions contribute to this shift.
How Maternal Transmission Works
Maternal influences reach infants via four routes. During birth and contact, maternal gut strains seed the newborn. Human milk oligosaccharides (HMOs) feed bacteria and direct metabolism. In mature milk, HMOs are present at approximately 5–20 g/L and reach the infant gut largely undigested to feed specialized taxa. Milk immunoglobulins, especially secretory immunoglobulin A (IgA), plus antimicrobial peptides and metabolites shape communities while training mucosal immunity. Colostrum contains the highest SIgA concentrations, which decline over the first month as the infant’s own production matures. Because milk is low-biomass, results from milk microbiota studies can be confounded by skin or environmental DNA. Maternal diet, medications, and exposures reconfigure the maternal microbiome and molecular cargo delivered to infants.
Key pathways explain these effects. Goblet cell-associated antigen passage (GAP) ferries antigens to immune cells, promoting tolerance. Short-chain fatty acids (SCFAs) engage G-protein-coupled receptors and epigenetic programs. Aryl hydrocarbon receptor (AhR) ligands from diet-microbe interactions support barrier integrity and immune balance.
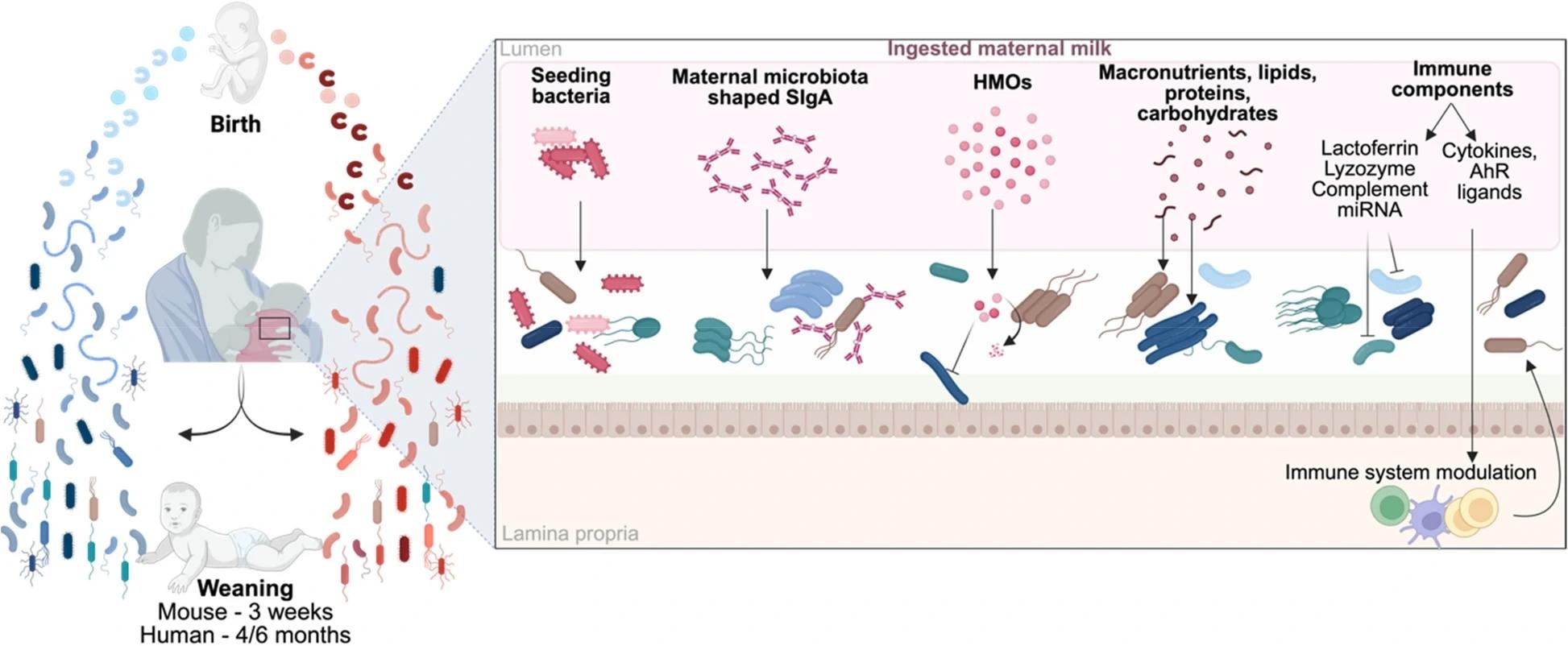
Impact of maternal milk components on microbiota establishment. Maternal milk plays a crucial role in the development of the offspring's microbiota, providing a range of components that influence bacterial growth and colonization. It contributes to the infant’s microbiota by supplying bacteria, SIgA, HMOs, nutrients, and immune factors, which together promote or inhibit the expansion of specific bacterial populations through both direct and indirect mechanisms. The interplay of these factors shapes the overall development of the microbiota in the offspring. SIgA, secretory immunoglobulin A; HMOs, human milk oligosaccharides; miRNA, microRNA; AhR, aryl hydrocarbon receptor
Feeding Choices and Microbial Trajectories
Breastfeeding delivers HMOs and secretory IgA that drive a microbial profile associated with immune training. When breastfeeding is not possible or is combined with formula, targeted innovations can narrow the gap. In a clinical study, infant formula supplemented with five HMOs shifted microbiome composition and function closer to that of breastfed infants. Probiotic-enriched formulas can also modulate microbial communities and metabolic outputs. These approaches are promising, though the durability of benefits and the specific taxa and pathways responsible still require definition to guide precise formulations.
Delivery, Antibiotics, and Early Perturbations
Mode of birth and early antibiotic exposure can perturb colonization patterns. Population studies link cesarean delivery and infant antibiotics with higher risks of asthma, celiac disease, inflammatory bowel disease, obesity, and even later metabolic alterations. The likely mediators include altered early colonizers, reduced transfer of beneficial maternal strains, and disrupted SCFA production. While causality can be complex, these findings underscore the need for stewardship of perinatal antibiotics and support for microbial recovery when treatment is necessary.
Maternal Diet: A Daily, Modifiable Lever
Maternal diet is a consistent, actionable influence on infant microbiome development. Diets high in fiber supply substrates for SCFA production, supporting epithelial health and immune tolerance. High-fat or ultra-processed patterns can skew community structure and microbial metabolites toward inflammation. Evidence from human cohorts and animal models shows that maternal dietary patterns reshape infant microbiota and immune programming, with effects that can persist. Notably, interventions such as maternal fiber optimization and avoidance of certain additives may protect the early microbial ecosystem, though findings for emulsifiers and non-nutritive sweeteners are primarily preclinical.
Transgenerational Signals and Today’s Children
A striking insight is that modern dietary shifts beginning in the 1970s impose both direct and transgenerational pressures. Today’s children may experience cumulative effects, resulting from their own exposure to modern diets combined with inherited influences from parental consumption, producing distinct phenotypes across generations. This concept reframes prevention as family-wide and time-layered, not infant-only. Understanding and mitigating these pressures is essential for public health strategies that preserve beneficial microbial ecosystems in early life. While maternal transmission dominates, the review briefly mentions that paternal microbiomes and diet may also contribute, though this area remains exploratory.
From Mechanism to Prevention
Promising levers include: supporting breastfeeding where feasible, designing formulas with evidence-based HMOs and probiotics when needed, prudent perinatal antibiotic use, maternal dietary guidance emphasizing fiber-rich, minimally processed foods, and clinical pathways that identify and repair early microbiome disruptions. Translating mechanisms such as GAP-mediated antigen delivery, SCFA signaling, and AhR modulation into practical interventions can move care from reactive to preventive. Longitudinal trials that track microbial, immune, and clinical outcomes are the next step in confirming the durability and equity of benefits across diverse populations. Effects are likely to be strain-, timing-, and context-specific, and precise targets remain to be identified.
Conclusions
The review demonstrates that maternal microbial transfer, HMOs, IgA, and the maternal environment collectively shape infant microbiome trajectories, which influence the lifetime risk of allergies, obesity, type 2 diabetes mellitus, and inflammatory bowel disease. Interventions such as breastfeeding support, evidence-based infant formula design, dietary counseling during pregnancy, and careful antibiotic stewardship can promote favorable colonization and immune education. Because modern diet patterns exert cumulative, transgenerational pressures, prevention should span families and generations. Priority research includes long-term, diverse trials that map microbial functions to clinical endpoints and test scalable strategies to preserve early microbial ecosystems.