For the first time, U.S. infants saw dramatically fewer RSV hospitalizations thanks to new maternal vaccines and nirsevimab, highlighting the urgent need for early, widespread prevention each season.
 Study: Interim Evaluation of Respiratory Syncytial Virus Hospitalization Rates Among Infants and Young Children After Introduction of Respiratory Syncytial Virus Prevention Products — United States, October 2024–February 2025. Image Credit: Jarun Ontakrai / Shutterstock
Study: Interim Evaluation of Respiratory Syncytial Virus Hospitalization Rates Among Infants and Young Children After Introduction of Respiratory Syncytial Virus Prevention Products — United States, October 2024–February 2025. Image Credit: Jarun Ontakrai / Shutterstock
In a recent report published in the Morbidity and Mortality Weekly Report, a group of researchers evaluated changes in respiratory syncytial virus (RSV)-associated hospitalization rates among children under five following the widespread availability of maternal vaccination and nirsevimab during the 2024-2025 season.
Because this was an ecologic analysis, the researchers did not have individual-level data linking RSV prevention product receipt to hospitalization outcomes. Therefore, causality cannot be definitively established.
Background
RSV is the leading cause of infant hospitalizations in the United States (US), with the highest risk among those under two months old. Each RSV season strains pediatric health systems, especially during peak winter months. Until recently, preventive options for RSV were limited. In 2023, two novel interventions became available: a maternal RSV vaccine administered during late pregnancy and nirsevimab, a long-acting monoclonal antibody for infants. These tools are aimed at protecting infants during their most vulnerable period. However, real-world data on their population-level effectiveness remains limited. The report emphasizes the need for further research to assess long-term and equitable outcomes, and notes several limitations, including the possibility of incomplete adjustments for underdetection or underenrollment, the non-national representativeness of the surveillance areas, and the interim nature of the data.
About the study
This analysis used surveillance data from two national systems: the RSV-Associated Hospitalization Surveillance Network (RSV-NET) and the New Vaccine Surveillance Network (NVSN). Both systems conducted active population-based surveillance for laboratory-confirmed RSV-associated hospitalizations in children under five. RSV-NET operated across 13 states, covering 161 counties, while NVSN covered seven metropolitan areas.
Hospitalization rates during October 2024 to February 2025 were compared to pooled pre-coronavirus disease 2019 (COVID-19) seasons (2018-2020). Children were categorized into three age groups: 0-7 months (eligible for maternal vaccination or nirsevimab), 8-19 months (some eligible for nirsevimab based on risk), and 20-59 months (ineligible). RSV confirmation was based on reverse transcription–polymerase chain reaction or rapid antigen detection within 14 days of admission. Rates were adjusted for factors such as under-testing, test sensitivity, hospital market share, and enrollment gaps. Sensitivity analyses were conducted, including one excluding Houston, Texas, where early virus circulation preceded widespread product use. Statistical analyses included rate ratios, Z-tests, and bootstrap-derived confidence intervals. This activity was reviewed by the Centers for Disease Control and Prevention and deemed public health surveillance, not requiring institutional review board approval.
Study results
A total of 18,389 RSV-associated hospitalizations were recorded: 11,681 during 2018-2020 and 6,708 during 2024-2025. Median patient age increased across both networks, indicating that younger infants were better protected in the later period.
Among infants aged 0-7 months, the primary target group for prevention, the 2024-2025 season saw significantly lower hospitalization rates. In RSV-NET, the rate dropped from 15.0 to 8.5 per 1,000 children, a 43% reduction. In NVSN, the rate fell from 14.8 to 10.7, reflecting a 28% reduction. The most substantial improvement was seen in infants aged 0-2 months, with reductions of 52% in RSV-NET and 45% in NVSN. When Houston was excluded from the NVSN analysis, the decline reached 71% for infants aged 0-2 months.
Children aged 8-19 months and 20-59 months, who were generally ineligible or partially eligible for RSV prevention, experienced higher hospitalization rates in 2024-2025 compared to pre-pandemic seasons. For example, RSV-NET showed a 33% increase among 8–19-month-olds and a 64% increase among 20-59-month-olds. These findings suggest the 2024–2025 RSV season was more severe overall and highlight that reductions among younger infants were likely due to the prevention products, not lower virus circulation or changes in care-seeking.
Trends were consistent across both weekly (RSV-NET) and monthly (NVSN) timeframes, particularly during peak months from December to February. This timing aligns with increased uptake of maternal RSV vaccination and nirsevimab. By February 2025, an estimated 66% of infants aged 0-7 months were protected, up from 30% in October.
The findings support the effectiveness of both prevention methods in reducing severe disease during peak transmission. In areas where products were deployed before the virus surge, like those excluding Houston, the benefits appeared even greater. These trends mirror results from European studies, further validating their global relevance.
The authors caution that observed reductions in hospitalizations may be underestimated given the increased severity of the 2024–2025 season for older, unprotected children, and recommend that population-level impact may be maximized through early, widespread deployment of RSV prevention products, before peak virus transmission.
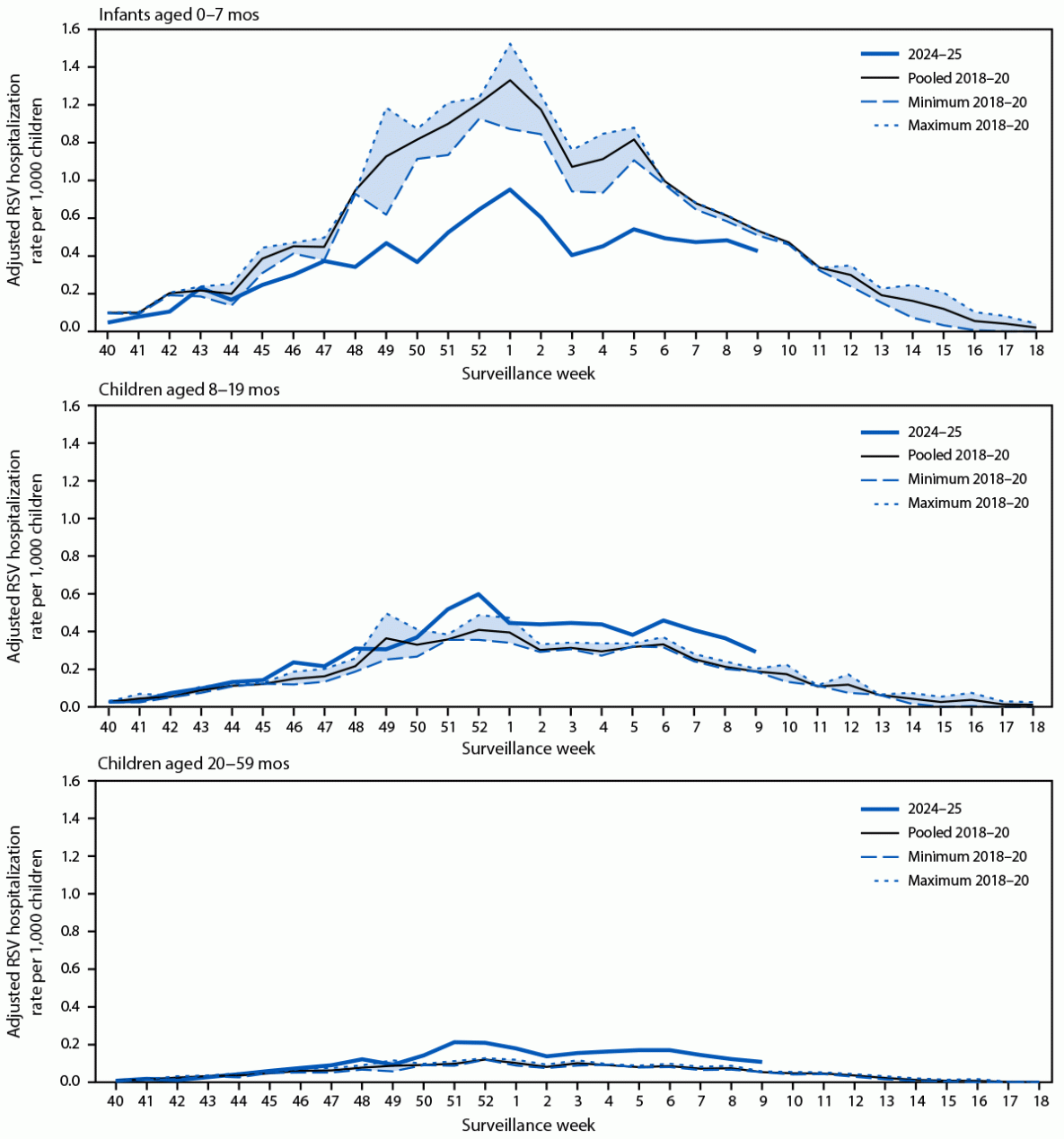
Respiratory syncytial virus–associated hospitalization rates* among children aged <5 years, by age group and surveillance week — Respiratory Syncytial Virus–Associated Hospitalization Surveillance Network, United States, October–April 2018–20 and October–February 2024–25
Conclusions
To summarize, the first U.S. RSV season with broad access to maternal vaccination and nirsevimab resulted in significantly fewer hospitalizations among infants aged 0-7 months. These declines were most notable during peak winter months and among the youngest infants, who face the highest risk of complications. Higher hospitalization rates in older, unprotected age groups reinforce that the reductions were likely due to the preventive products. These findings highlight the urgency of early and widespread use of maternal RSV vaccines and nirsevimab. Health systems must prioritize the timely delivery of these interventions to protect infants in future RSV seasons and reduce the overall disease burden.
Because individual-level vaccination or prophylaxis status was unavailable, the report emphasizes the need for continued surveillance and further research to determine these preventive strategies' long-term and equitable effectiveness.
Journal reference:
- Patton ME, Moline HL, Whitaker M, et al. Interim Evaluation of Respiratory Syncytial Virus Hospitalization Rates Among Infants and Young Children After Introduction of Respiratory Syncytial Virus Prevention Products - United States, October 2024-February 2025. MMWR Morb Mortal Wkly Rep. (2025), doi: 10.15585/mmwr.mm7416a1, https://www.cdc.gov/mmwr/volumes/74/wr/mm7416a1.htm