Nuclear magnetic resonance (NMR) is a structurally rich and non-intrusive technique that is quantitative by nature. These inherent characteristics make NMR extremely robust for identifying and characterizing reaction intermediates, thus allowing a better understanding about the kinetics of chemical reactions and reaction mechanisms.
lthough standard NMR tubes were used to analyze many chemical transformations over the years, chemical and physical restrictions reduce the types of reactions to which this type of NMR reaction monitoring is relevant. Since the parameters such as mixing, pressure and temperature, are not controlled, they can have considerable effects on the reaction rate. These limitations can now be reduced with the use of online reaction monitoring1.
This article shows the complementarity of infrared (IR) and NMR spectroscopy and demonstrates how NMR can be applied to enhance the robustness and understanding of the synthesis of API (active pharmaceutical ingredient) precursor.
Results and discussion
The first step of a telescoped reaction for API formation2 is the reaction between 3-methylhexanoic acid (I) and Meldrum’s acid (II) and pivaloyl chloride (III) which results in the formation of Meldrum’s acid adduct (IV). This initial step cannot be studied with GC-MS and LC-UV, which are traditional analytical technology methods, because the products, intermediates, and reagents not only lack strong chromophores but also readily decompose with temperature.
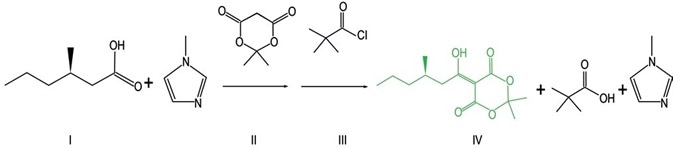
Figure 1
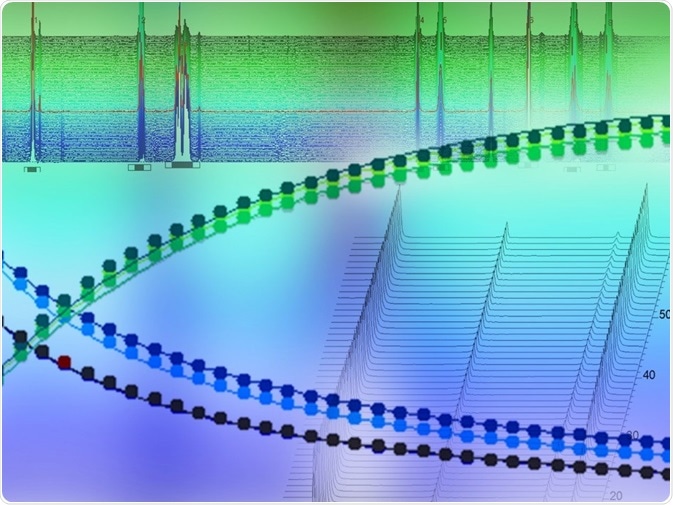
As a result, 1D 1H NMR spectroscopy was used to study the course of the reaction to resolve these limitations. The presence of an unknown peak was revealed by 1H NMR experiments, this peak was abundantly present at the onset of the reaction.
FT-IR was used to observe an unknown peak, which first increased and then decreased during the course of the reaction, revealing the usual behavior of an intermediate. However, the IR data were not enough to completely explain the intermediate structure and hence, it could not be measured. The unknown peak was identified to be pivalic anhydride by using a combination of 2D NMR and mass spectrometry.
The fact that the reaction was not controlled by dose together with the existence of the pivalic anhydride intermediate led to the conclusion that a second mechanistic pathway, path B, (Figure 2) should definitely exist.
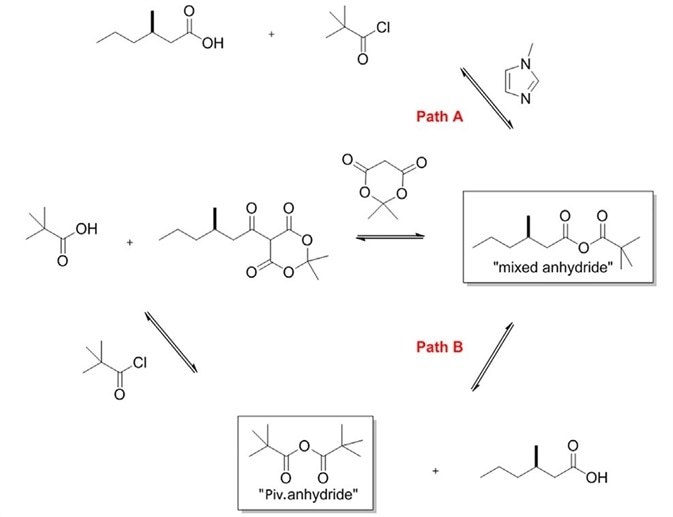
Figure 2. Proposed reaction mechanism, showing the mixed anhydride intermediate in pathway A and the pivalic anhydride, explained by mechanistic pathway B.
Online NMR reaction monitoring3 was used to track a similar reaction with commercial 3-methylpentanoic acid, instead of 3-methylhexanoic acid. This was done by placing a reaction vessel beside the NMR spectrometer and allowing the reaction mixture to flow via the magnet (Figure 3).
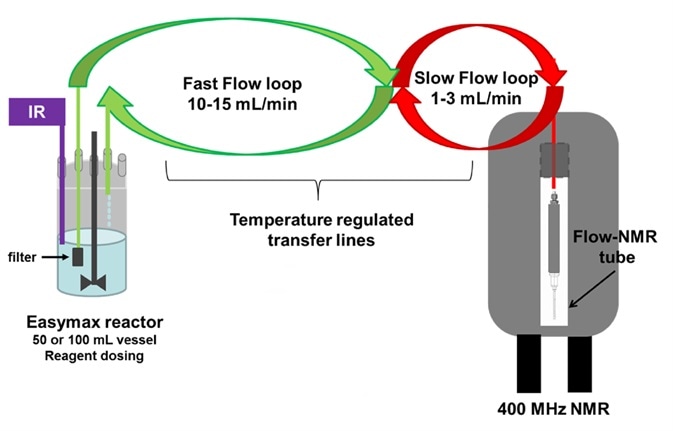
Figure 3. Laboratory set up illustrating how the reaction mixture is pumped from the vessel, though temperature regulated transfer lines, to the flow tube4, 5 placed in the coil area of the NMR probe.
The complementarity of these methods, that is NMR and infrared (IR) spectroscopy, is illustrated by the comparison of online, inline FT-IR and NMR monitoring data (Figures 4 and 5). In-situ FT-IR measurements were carried out that provided high-resolution data from the onset of the reaction. FT-IR data, however, is not quantitative and it is not adequate to explain the complete structure of the main reaction intermediate.
Although quantitative data is provided by inline (standard tube) NMR, no information is provided on reaction initiation. It was possible to acquire online (flow) NMR data much closer to the initiation of reaction, and the presence of early formation of intermediates was visualized, initially increasing and later decreasing in parallel with the behavior perceived by FT-IR.
Experiment
Using an EasyMax reaction vessel (Mettler-Toledo) at 25°C, the reaction was performed in acetonitrile. Bruker InsightMR flow tube5 was then used to carry out online reaction monitoring. A Julabo FP-50-HE heating circulator filled with Syltherm XLT temperature regulation fluid was also used to regulate the temperature of the transfer lines.
The rate of flow through the system was 3 mL/minute and this was regulated with a dual piston pump (Lab Alliance Prep 100, Scientific Systems, Inc. State College, PA, USA). Next, spectra were obtained on the flowing solution on a Bruker 600 MHz AVANCE III NMR spectrometer equipped with a TXO probe, or a Bruker 400 MHz AVANCE III fitted with a BBFO probe. 1H NMR spectra were obtained with 30° pulse angle, four scans, and 10–second relaxation delay. Dynamics Center 2.2.4 was finally used to analyze the data.
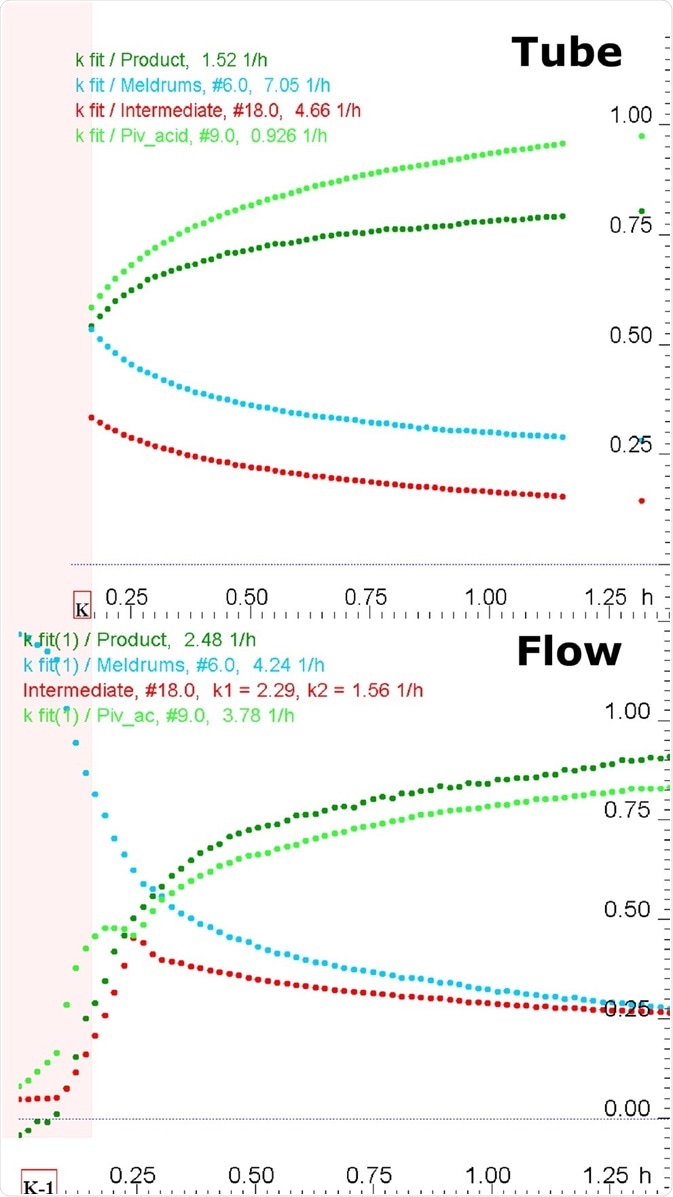
Figure 4. Kinetics profiles (equivalents vs time) obtained from inline (standard NMR tube, top) and online (flow, bottom) NMR measurements.
Conclusions
NMR online reaction monitoring allows a chemist to:
- Acquire kinetic data of chemical processes much closer to the initiation of reaction
- Concurrently track the reaction by NMR as well as other techniques such as pH, UV, IR, and MS
- Collect real-time quantitative information under lab conditions of pressure, temperature, and stirring
Good structural and mechanistic information is obtained through studies in static NMR tubes, especially for reactive or labile intermediates. However, care should be taken when depending on kinetic information obtained from systems that lack sufficient mixing1.
In the laboratory, IR spectroscopy is complemented by NMR reaction monitoring. This improves process understanding and supports in-situ IR process monitoring in pilot as well as manufacturing plants.
![Mid-IR kinetic profile [absorbance at 1810 wn vs time (h)] for the pivalic anhydride intermediate.](https://d2jx2rerrg6sh3.cloudfront.net/image-handler/picture/2017/8/Figure_5.jpg)
Figure 5. Mid-IR kinetic profile [absorbance at 1810 wn vs time (h)] for the pivalic anhydride intermediate.
References
- Foley D. A., Dunn A. L., Zell M. T., Magn. Reson. Chem., 2015, DOI: 10.1002/mrc4259
- Clegg, I. M., Gordon C. M., Smith D. S., Alzaga R., Codina A.; Anal. Methods, 2012, 4, 1498-1506
- Dunn A. L., Codina A., Foley D. A., Marquez B. L., Zell M. T., Magn. Reson. Chem., 2015, DOI: 10.1002/mrc.4317
- Foley D. A., Bez, E., Codina A., Colson K. L., Fey M., Krull R, Piroli D., Zell, M. T., Marquez, B. L., Anal. Chem., 2014, 86, 12008
- www.bruker.com/insightMR, accessed 07 Nov 15
About Bruker BioSpin - NMR, EPR and Imaging

Bruker BioSpin offers the world's most comprehensive range of NMR and EPR spectroscopy and preclinical research tools. Bruker BioSpin develops, manufactures and supplies technology to research establishments, commercial enterprises and multi-national corporations across countless industries and fields of expertise.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.