The full-length human TL1A (Uniprot: O95150-1) is expressed by the HEK293/Human TL1A Stable Cell Line. Using flow cytometry, the surface expression of human TL1A was verified.
Product details
- Complete traceable record, strict quality control, and verified stability of cell passage
- Commercially licensed parental cell line that was lawfully acquired from a globally renowned cell resource bank
- Global commercial license assistance whenever a regulatory filing is required
- Genetically altered cell lines best represent MOA (Mechanism of Action)
- Increased activity and a longer assay window for a reliable and repeatable cell-based bioassay
- Extensive application data to aid in the development and validation of assays
Application
Useful for cell-based TL1A binding assay.
Growth properties
Adherent
Selection marker
Puromycin (2 μg/mL)
Complete growth medium
DMEM + 10 % FBS
Freeze medium
Serum-free cell cryopreservation medium.
Quantity
One vial contains at least 5 × 106 cells in 1 mL serum-free cryopreservation medium.
Storage
Frozen in liquid nitrogen.
Mycoplasma testing
Negative
Sterility testing
Negative
Instructions for use
The data sheet provides detailed protocols for culturing and assay.
ACRO Quality Management System
- Quality Control Process
- QMS (ISO, GMP)
- Quality Advantages
Performance data
Receptor assay
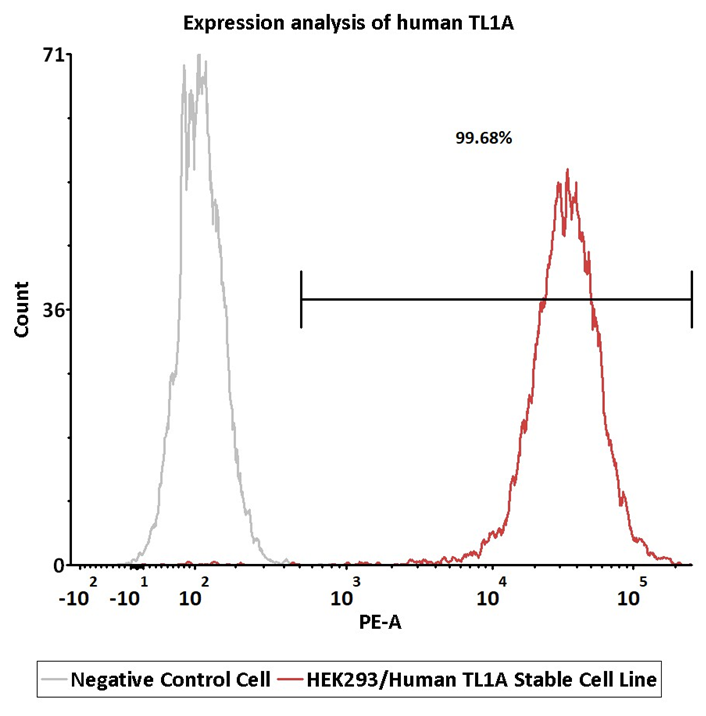
Expression analysis of human TL1A on HEK293/Human TL1A Stable Cell Line by FACS. Cell surface staining was performed on HEK293/Human TL1A Stable Cell Line or negative control cell using anti-human TL1A Antibody, followed by staining with PE anti-human IgG Fc Antibody. Image Credit: ACROBiosystems
Passage stability
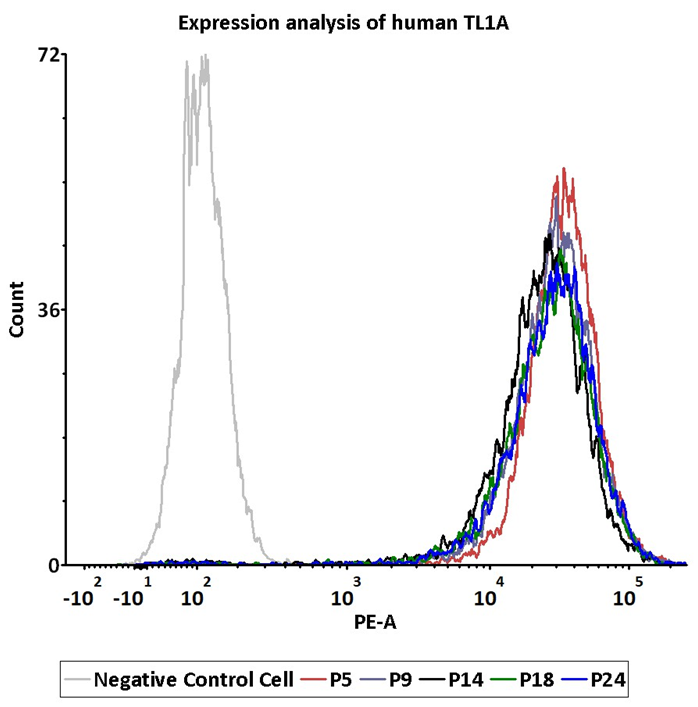
Passage stability analysis of human TL1A expression by FACS. Flow cytometry surface staining of human TL1A on HEK293/Human TL1A Stable Cell Line demonstrates consistent mean fluorescent intensity across passage 5-24. Image Credit: ACROBiosystems