ACROBiosystem's SAFENSURE™ Recombinant Factor C Endotoxin Detection Kit is a novel endotoxin detection kit powered by recombinant Factor C technology.
Comparable to LAL method - The endpoint fluorescent assay is comparable to other chromogenic quantitative LAL methods.
High specificity - In contrast to the LAL Assay, the absence of Factor G in the rFC test kit means that false-positive results caused by β-glucan activation are not anticipated.
Accuracy - The traceability of endotoxin standards within the kit is aligned with the USP Standard (Catalog No: 1235503).
Fast time to results - Results in just an hour.
High sensitivity - A sensitivity range of 0.005 to 5.0 EU/mL.
Extensive validation - Verification of multiplex biological products, various types of microplate readers, and different buffer systems, along with a thorough validation of specificity, sensitivity, precision, accuracy, applicability, and other factors in accordance with the parameters outlined in the EUROPEAN PHARMACOPOEIA 11.0, USP chapter <<1225>>, and <86>.
Sustainable resource - Eliminate reliance on animal-sourced reagents, lessen dependence on horseshoe crab resources and fishing, and ensure a long-term supply.
Good inter-batch consistency - Product batch consistency is assured through the use of genetic recombination technology in manufacturing.
Recombinant C protein: Saving horseshoe crabs for a greener future

Image Credit: ACROBiosystems
Product overview
The Recombinant Factor C Endotoxin Detection Kit represents an innovative method for endotoxin detection that uses recombinant technology. Recombinant Factor C, which is the initial component in the coagulation cascade reaction of the horseshoe crab, becomes activated in the presence of endotoxin.
Once activated, Factor C is capable of cleaving a fluorogenic substrate, resulting in the generation of a fluorescent signal. The increase in fluorescence is directly proportional to the amount of endotoxin present.
The experiment is conducted on a white 96-well plate, with measurements taken at the start and after a one-hour incubation at 37 ° C. To ascertain whether the sample is contaminated with endotoxin, a fluorescence microplate reader should be employed, measuring at an ex/em wavelength of 380/440 nm.
Assay principles
The Recombinant Factor C Endotoxin Detection Kit signifies a groundbreaking approach to endotoxin detection that employs recombinant technology. Recombinant Factor C, the primary element in the coagulation cascade reaction of horseshoe crabs, is activated in the presence of endotoxin. Upon activation, Factor C can cleave a fluorogenic substrate, leading to the release of a fluorescent signal.
The increase in fluorescence correlates directly with the quantity of endotoxin present. The experiment is performed using a white 96-well plate, with measurements taken initially and after a one-hour incubation at 37 ° C. To determine if the sample is contaminated with endotoxin, a fluorescence microplate reader should be used, measuring at an ex/em wavelength of 380/440 nm.
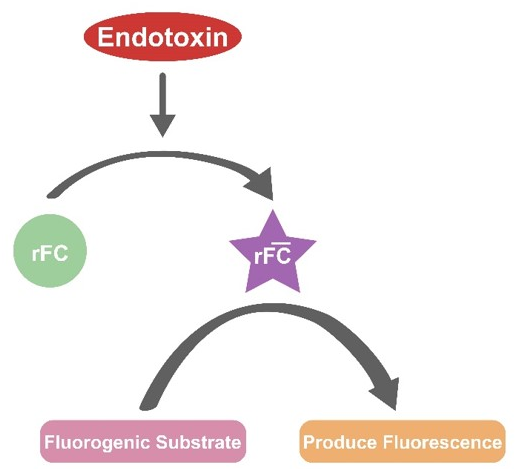
Image Credit: ACROBiosystems
Materials provided
Source: ACROBiosystems
| ID |
Components |
Size |
| RES056-C01 |
Bacterial Endotoxin Standard |
1 vial |
| RES056-C02 |
Recombinant Factor C Protein |
48 tests/96 tests |
| RES056-C03 |
Fluorogenic Substrate |
48 tests/96 tests |
| RES056-C04 |
Water for Bacterial Endotoxins Test |
50 mL |
| RES056-C05 |
96 Well Endotoxin Free Black Plate |
1 plate |
Application
Production process (Process control)
- Intermediate product testing
- Raw materials
- Water testing
Final product (Release testing)
- Medical devices
- Parenteral drugs and biological products
- Infusion, injection, or transfusion cells
- Cell culture media
It is for research use only.
Storage
- Store the unopened kit at 2-8 ° C upon receipt.
- Identify the expiration date on the external packaging and refrain from using reagents that have surpassed their expiration date.
ACRO Quality Management System
- Quality Control Process
- QMS (ISO, GMP)
- Quality Advantages
Performance data
Validation report
SAFENSURE™ Recombinant Factor C Endotoxin Detection Kit has been extensively validated, adhering to EP 11.0 and USP <1225><86>.

Image Credit: ACROBiosystems
Validation included multiplex biological products, multiple microplate readers, and diverse buffer systems, with thorough verification of specificity, sensitivity, precision, accuracy, and applicability, following the parameters outlined in the EUROPEAN PHARMACOPOEIA 11.0 and USP chapter <1225><86>.
Bioactivity-Fluorescence
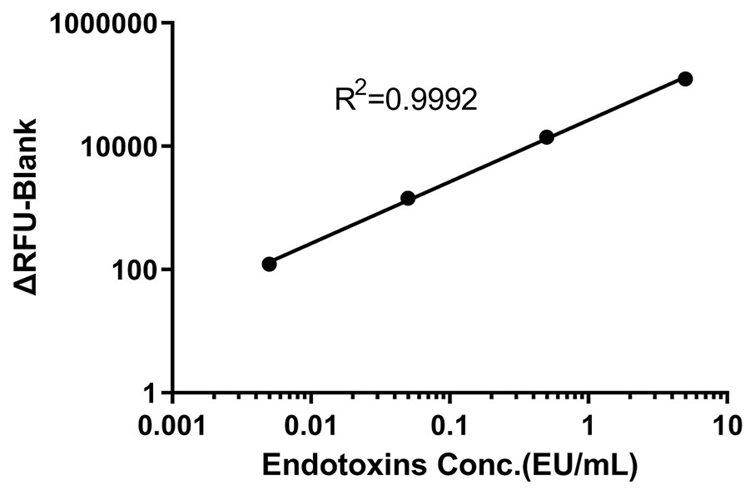
Take the logarithm of the concentration of the Endotoxin working standard solution as the abscissa, and take the ΔRFU as the ordinate. Fitting the standard curve with a linear model, and the correlation coefficient R should be ≥ 0.98 (QC tested). Image Credit: ACROBiosystems
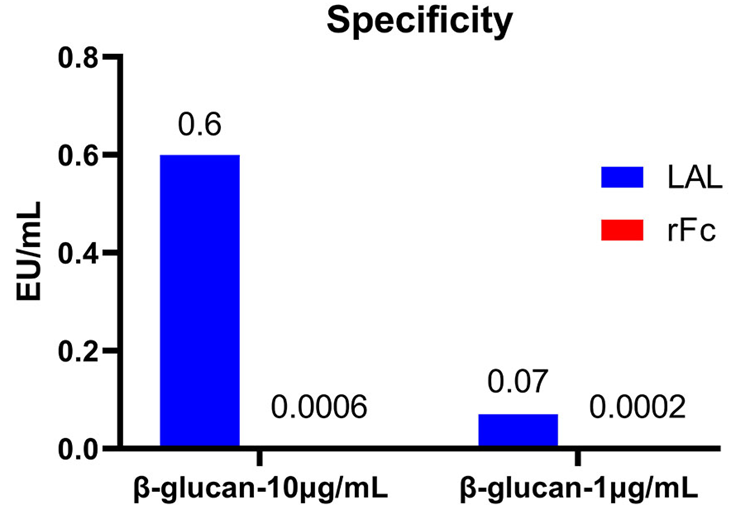
The rFC method was employed to detect endotoxin residues in β-glucan at concentrations of 10 µg/mL and 1 µg/mL. No non-specific signals were detected. In contrast, the dynamic chromogenic method used for β-glucan detection resulted in the detection of endotoxin and non-specific signals. This indicates that recombinant factor C does not react with β-glucan, demonstrating the good specificity of the rFC method. Image Credit: ACROBiosystems