ACROBiosystem's SAFENSURE™ Sterility Rapid Detection Kit is a validated NAT-based sterility rapid detection kit designed to accelerate in-process and lot-release testing.
Product overview
The Sterility Rapid Detection Kit (using NAT) has been developed and validated to comply with the standards outlined in European Pharmacopeia Chapter 2.6.1 and USP General Chapter 71 requirements to enhance the efficiency of in-process and lot-release sterility testing.
A significant benefit of this kit is its integrated detection capability. When used in conjunction with the SAFENSURE™ Sterility Sample Preparation Kit (OPA-E102), it effectively and reliably identifies both bacteria and fungi simultaneously in biological products, pharmaceuticals, and medical devices - eliminating the need for separate qPCR assays.
This integration minimizes the handling of split samples and reduces the frequency of operations, lowering the risk of errors.
It is a replacement for the conventional 14-day culture-based methods by adhering to stringent sterility testing regulations (ensuring no detectable viable microorganisms). Despite its dual detection capability, it provides precise results in merely three hours, improving product release efficiency and decreasing testing time and labor costs.
Features
Broad coverage: It encompasses microbial species, including those identified in prominent Pharmacopoeias (EP, USP, ChP).
Simultaneous bacteria-fungi detection: This kit enables the simultaneous detection of bacteria and fungi without requiring separate qPCR assays, thereby simplifying sterility testing processes and reducing operational complexity.
Strong specificity: The product is formulated using various primers and probes specifically targeting bacteria and fungi, ensuring no cross-reactivity with closely related non-bacterial and non-fungal strains.
High sensitivity: Completely adheres to or exceeds the regulatory standard of 100 CFU/mL.
Convenient operation: Intended for testing a single well, the kit includes all necessary components for straightforward usage.
Comparable results: The results obtained from the kit are comparable to those derived from culture-based methods.
High-quality: The kit is manufactured in a facility that complies with the ISO 13485 standard.
Application
The kit is used for identifying bacterial or fungal contamination in cells and bioproduct media. It is intended solely for quality control and manufacturing processes. This product is designated for research purposes only.
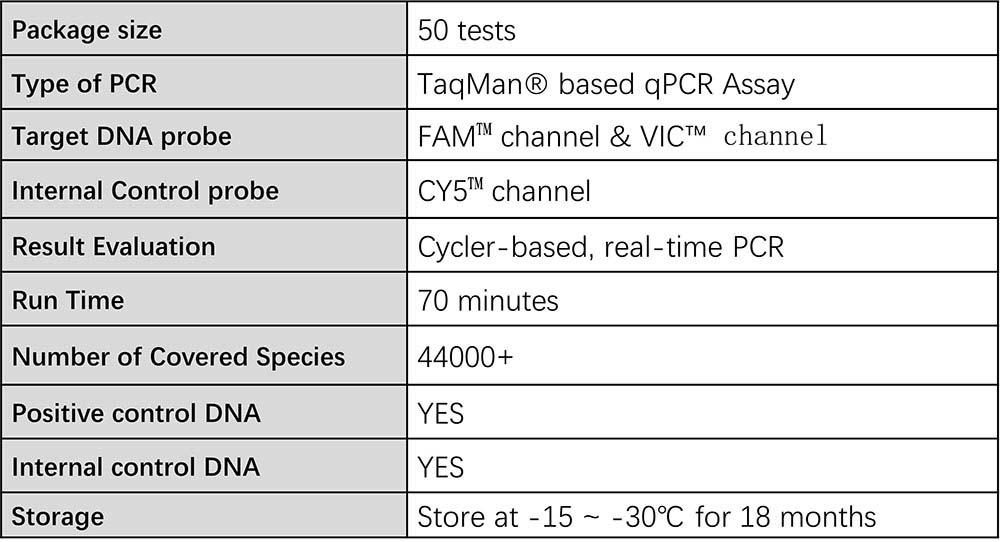
Technical specifications
Source: ACROBiosystems
Attention
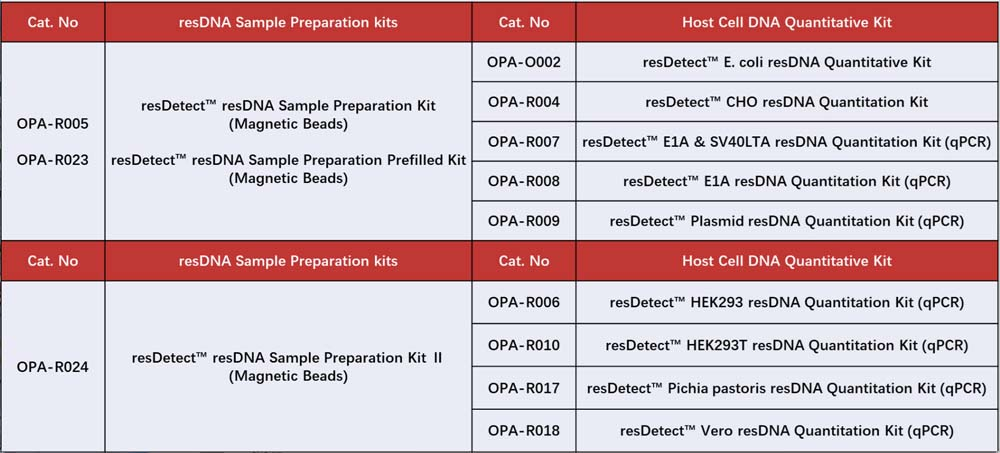
If the experimental workflow involves the preparation of host cell DNA samples, please use the suggested sample preparation kits outlined in the table. This guarantees that the buffers employed during both sample preparation and residual DNA quantification are uniform, thereby facilitating dependable and precise results.
Source: ACROBiosystems
Materials provided
Source: ACROBiosystems
| ID |
Components |
Size |
| OPA-S103-01 |
Sterility Assay Mix |
200 μL |
| OPA-S103-02 |
Sterility qPCR Master Mix |
400 μL |
| OPA-S103-03 |
Sterility Positive Control |
500 μL |
| OPA-S103-04 |
Sterility Internal Control |
200 μL |
| OPA-S103-05 |
DNase/RNase-Free Water |
1 mL |
ACRO Quality Management System
- Quality Control Process
- QMS (ISO, GMP)
- Quality Advantages
Performance data
Typical data
High Sensitivity: All 10 tests per species were positive, exceeding or meeting the 100 CFU/mL guideline.
Source: ACROBiosystems
|
Strain Species
|
Limit (CFU/mL)
|
Results
|
|
Bacillus spizizenii
|
100
|
10/10
|
|
Burkholderia cepacian
|
100
|
10/10
|
|
Clostridium sporogenes
|
100
|
10/10
|
|
Cutibacterium acnes
|
100
|
10/10
|
|
Escherichia coli
|
100
|
10/10
|
|
Micrococcus luteus
|
100
|
10/10
|
|
Pseudomonas aeruginosa
|
100
|
10/10
|
|
Staphylococcus aureus
|
100
|
10/10
|
|
Streptococcus pyogenes
|
100
|
10/10
|
|
Candida albicans
|
100
|
10/10
|
|
Aspergillus brasiliensis
|
100
|
10/10
|
|
Aspergillus niger
|
100
|
10/10
|
High Specificity: The three fluorescent channels are independent and exhibit no crosstalk, with a precision CV of ≤ 0.25% (n = 10).
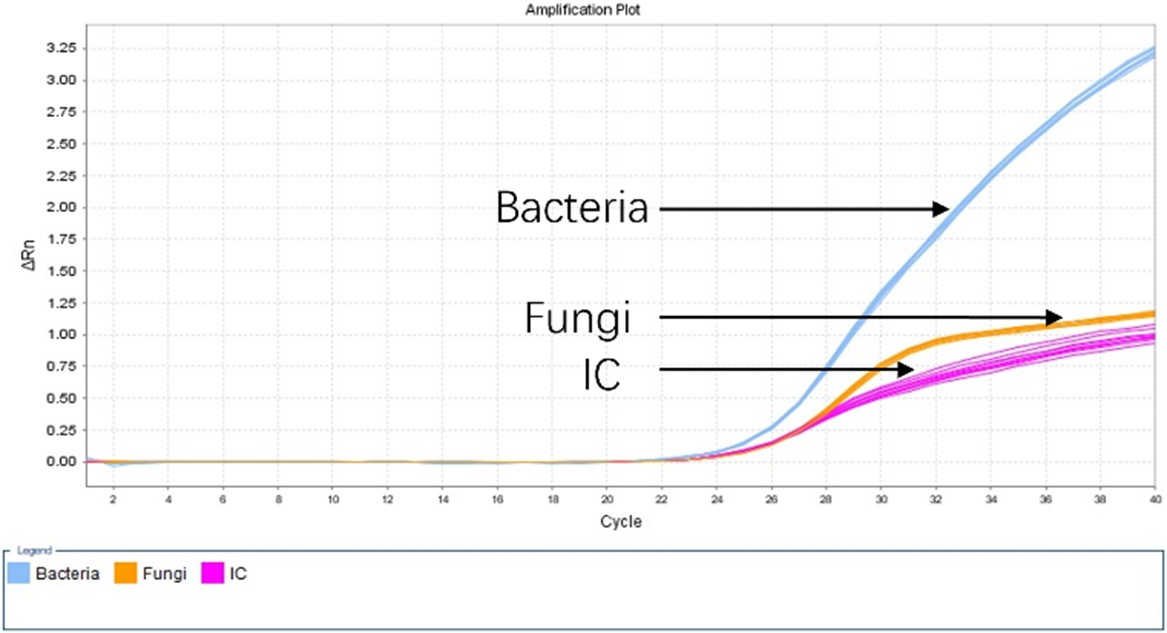
Image Credit: ACROBiosystems