SAFENSURE™ Mycoplasma Rapid Detection Kit (qPCR) is a validated NAT-based mycoplasma rapid detection kit designed to accelerate in-process and lot-release testing.
Broad coverage - The kit encompasses more than 250 species of Mollicutes (Mycoplasma, Acholeplasma, and Spiroplasma), which includes all mycoplasma species recognized in the Pharmacopoeias (EP, USP, JP)
Strong specificity - Created using various primers and probes aimed at the 16s rRNA of mycoplasma species, ensuring no cross-reactivity with closely related non-mycoplasma strains.
High sensitivity - SAFENSURE™ completely adheres to or exceeds the regulatory standard of 10 CFU/mL
Convenient operation - Created for testing a single well, the kit includes all necessary components for straightforward application.
Comparable results - The results obtained from the kit are comparable to those derived from culture-based methods.
High quality - This Kit is produced in a facility that adheres to GMP-like standards and complies with the ISO 13485 standard.
Product overview
To accelerate in-process and lot-release testing, the mycoplasma rapid detection kit has been developed and validated to comply with the standards specified in European Pharmacopeia Chapter 2.6. 7, using Nucleic Acid Amplification Technology (NAT).
When used in conjunction with the mycoplasma DNA Sample Preparation kit (OPA-E101), this kit effectively and reliably identifies mycoplasma contamination in biological products, adhering to or surpassing the regulatory guidance of 10 CFU/mL.
The assay removes the requirement for labor-intensive culture-based methods, streamlining the mycoplasma testing procedure and providing results within 2 to 2.5 hours with a high degree of efficiency.
Features
Broad coverage: Encompasses more than 250 species of Mollicutes (Mycoplasma, Acholeplasma, and Spiroplasma), which includes all mycoplasma species recognized in the Pharmacopoeias (EP, USP, JP).
Strong specificity: Created using various primers and probes aimed at the 16s rRNA of mycoplasma species, ensuring no cross-reactivity with closely related non-mycoplasma strains.
High sensitivity: Completely adherent to or exceeding the regulatory standard of 10 CFU/mL
Convenient operation: Intended for testing a single well, the kit includes all necessary components for straightforward application.
Comparable results: The results obtained from the kit are comparable to those derived from culture-based methods.
High-quality: This kit is manufactured in a facility that complies with the ISO 13485 standard.
Application
The kit is used for the identification of mycoplasma contamination in biological products, cells, and bioproduct media, among others. It is intended solely for quality control and manufacturing processes. This product is designated for research purposes only.
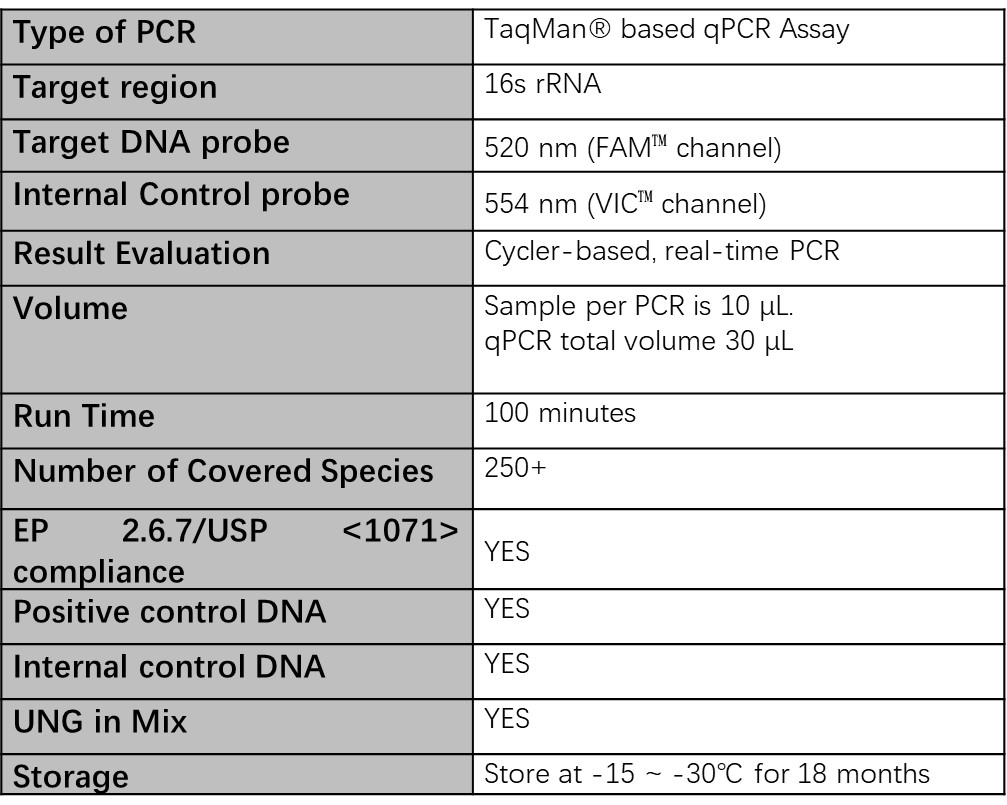
Technical specifications
Source: ACROBiosystems
Materials provided
Source: ACROBiosystems
| ID |
Components |
Size |
| OPA-S102-01 |
PC Powder |
1 tube |
| OPA-S102-02 |
Purple-capped empty tube |
1 tube |
| OPA-S102-03 |
DNA Dilution Buffer |
1.5 mL×2 |
| OPA-S102-04 |
Internal Control DNA |
200 μL×2 |
| OPA-S102-05 |
2×qPCR Master Mix |
400 μL×2 |
| OPA-S102-06 |
Myco Primer&Probe Mix |
200 μL |
| OPA-S102-07 |
Dnase/Rnase-Free Water |
1.5 mL×3 |
ACRO Quality Management System
- Quality Advantages
- QMS (ISO, GMP)
- Quality Control Process