Researchers in the United States have shown that water-soluble derivatives of vitamin E (α-tocopherol) exhibit potent antiviral activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
Kevin Harrod from the University of Alabama at Birmingham and colleagues found that the compounds synergized with the antiviral drug remdesivir to inhibit SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) – an enzyme that is crucial for transcription and replication of the viral genome.
Although intense research efforts to rapidly identify effective antiviral therapies have largely focused on repurposing existing drugs, only remdesivir has so far been authorized as a COVID-19 treatment, and its clinical benefits are modest.
“Here, we employed a novel artificial intelligence query of FDA-approved compounds for repurposing as antivirals against SARS-CoV-2 and identify tocopherol derivatives with potent antiviral activity and synergy with remdesivir,” writes Harrod and colleagues.
The team says the findings have important implications, given that many tocopherol derivatives are already considered safe for use in humans.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The need for broad-spectrum interventions
The emergence of several coronaviruses over recent years highlights the need for broad-spectrum interventional strategies.
Despite the unprecedented development and authorization of effective vaccines to protect against COVID-19, breakthrough SARS-CoV-2 infections, vaccine hesitancy, and the inequitable distribution of vaccines globally emphasize the need for therapeutic interventions that will limit severe disease and mortality.
Currently, the FDA-approved drug remdesivir is the only antiviral to have demonstrated improvement of clinical outcomes to date, albeit with limited effectiveness and the limitation of intravenous administration.
What did the researchers do?
Using an artificial-intelligence-driven in silico screen of FDA-approved compounds for repurposing as antivirals against SARS-CoV-2, the team identified almost 100 that they prioritized for in vitro screening.
This led to the identification of 12 compounds that reduced SARS-CoV-2 propagation by more than 90% at a concentration of just 10µM.
Next, the researchers found that five of these top 12 compounds reduced the burden of SARS-CoV-2 by more than 90% in the human lung epithelial cell line Calu3.
Four of these drugs – niclosamide, remdesivir, lopinavir, and D-α-tocopherol polyethylene glycol succinate (TPGS) – showed maximal efficacy in preventing established SARS-CoV-2 infection in VeroE6 cells.
In addition, 11 of the 12 top compounds demonstrated strong antiviral activity against the seasonal betacoronavirus OC43, suggesting that most of the compounds are effective against betacoronaviruses more broadly.
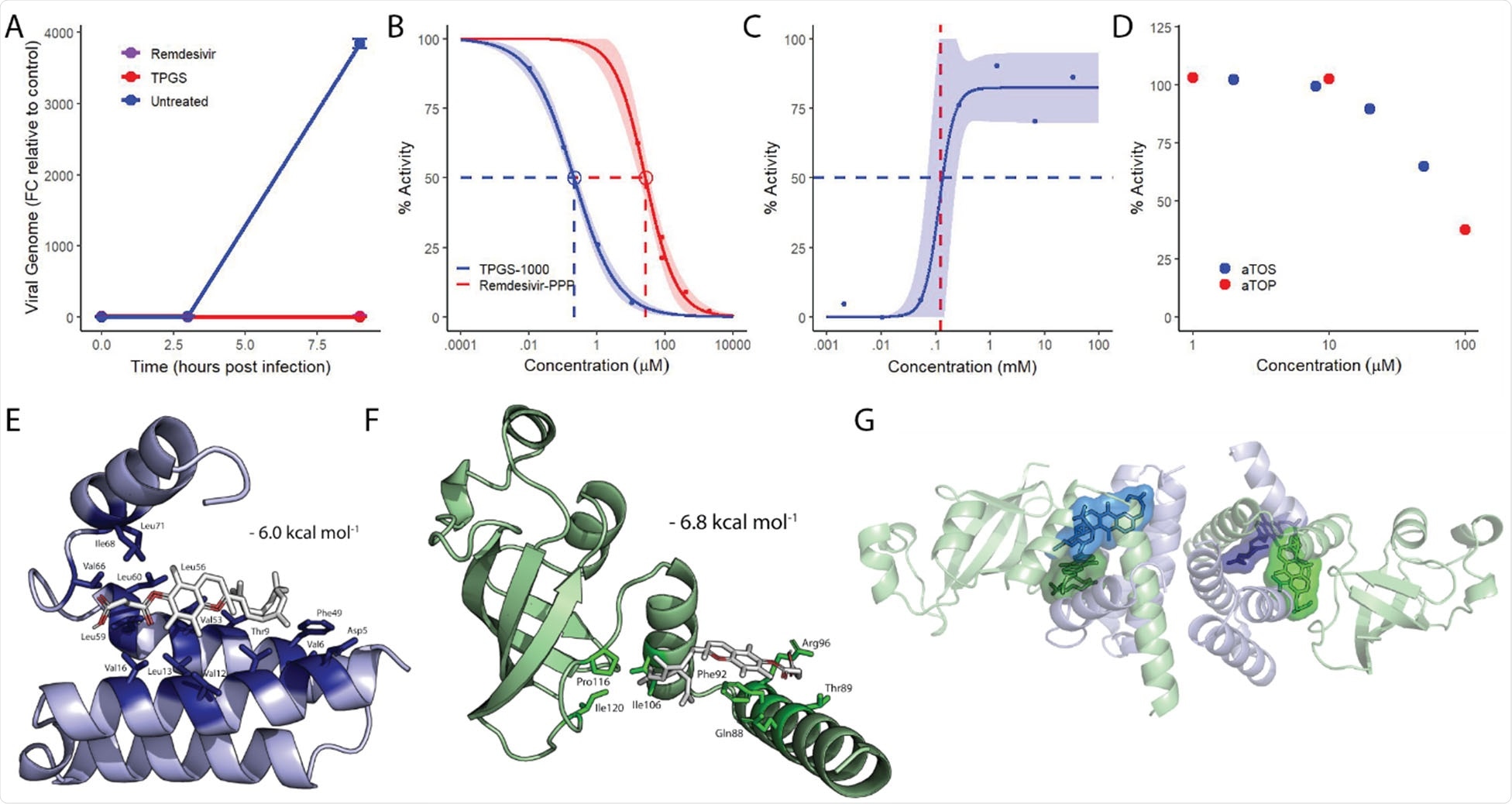
Water-soluble tocopherols inhibit the transcriptional activity of SARS-CoV-2 RNA-dependent RNA polymerase. A) Graph representing the fold change of SARS-CoV-2 genome in infected VeroE6 cells relative to initial uptake in the untreated control. Remdesivir and TPGS were given at a fully effective dose, 10 μM and 30 μM, respectively. Points and error bars represent the mean +/- SEM calculated from two technical replicates. B) Dose-response curves for the transcriptional activity of the SARS-CoV-2 replication complex treated with TPGS (blue) and remdesivir (red). The shaded region represents the 95% CI and points represent replicates from a single experiment. C) Dose-response curve for the transcriptional activity of the SARS-CoV- 2 replication complex treated with high concentrations of TPGS, with 50% activity (blue, dashed line) and the critical micelle concentration of TPGS (red, dashed line) indicated. The upper limit of the model was not fixed. Points represent replicates from a single experiment. D) Dose-response plot for increasing concentrations of αTOS (blue) and αTOP (red). Points represent replicates from a single experiment. E) Docking model of the most favorable pose for αTOS (white) interacting with conserved, hydrophobic residues (dark blue, named) within NSP7 (light blue). F) Docking model of the most favorable pose for αTOS (white) interacting with conserved, hydrophobic residues (dark green, named) within NSP8 (light green). G) Representation of the heterotetrameric structure of NSP7 (light blue) and NSP8 (light green) with the most favorable poses of αTOS interacting individually with each NSP7 (dark blue) and NSP 8 (dark green) superimposed.
Screening the compounds for synergistic combinations
When Harrod and colleagues screened the top compounds for synergistic combinations, they found that a combination of remdesivir and TPGS resulted in an 8-fold increase in antiviral potency.
To understand the mechanism underlying the antiviral activity of TPGS, the researchers tested its constituent components: D-α-tocopherol, succinate, and polyethylene glycol.
Since α-tocopherol alone is insoluble in an aqueous environment, the researchers instead tested α-tocopherol succinate (αTOS) and α-tocopherol phosphate (αTOP).
This revealed that αTOS is capable of inhibiting SARS-CoV2 replication in VeroE6 cells, suggesting that α-tocopherol is the active antiviral component in TPGS.
Given the potent synergy observed between TPGS and remdesivir – which is a known inhibitor of the SARS-CoV-2 RdRp – the team hypothesized that TPGS also inhibits this RdRp.
The researchers measured the ability of TPGS to inhibit the transcriptional activity of purified SARS-CoV-2 RdRp composed of the catalytic subunit non-structural protein 12 (NSP12) and two accessory proteins – NSP7 and NSP8.
They found that TPGS inhibited the transcriptional activity of the SARS-CoV-2 RdRp, with a potency that was approximately 100-fold that of remdesivir.
RdRp had high-affinity binding sites for αTOS
To further elucidate the interaction between the water-soluble tocopherols and the SARS-CoV-2 RdRp, the team performed computational docking studies across the individual components of the RdRp.
This identified high-affinity binding sites for αTOS within each of NSP7, NSP8, and NSP12.
Interestingly, while the top poses for αTOS were identified at a few surface locations across NSP12, all top poses for αTOS interacting with NSP7 and NSP8 localized to a single region within each protein, thereby providing greater confidence in their relevance.
In each case, the most favorable binding poses localized with residues seen in one of two recently described hydrophobic interfaces required for the assembly of functional RdRp.
“Taken together, these findings strongly support a mechanism by which TPGS prevents or destabilizes the assembly of the SARS-CoV-2 RdRp,” writes Harrod and colleagues.
TPGS as an effective antiviral against SARS-CoV-2
The researchers say the study has identified TPGS as an effective antiviral against SARS-CoV-2 and β-coronaviruses more broadly that also displays strong synergy with remdesivir.
“These findings are significant given that many tocopherol derivatives, including TPGS, are considered safe for humans, are orally bioavailable, and dramatically enhance the activity of the only approved antiviral for SARS-CoV-2 infection,” concludes the team.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.