Vaccination is critical against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but emerging mutant variants of the virus impair the effectiveness of vaccines based on the original/wildtype SARS-CoV-2. Consequently, bivalent vaccines with spike messenger ribonucleic acid (mRNA) of wildtype and Omicron BA.1 or BA.4/5 variant have been developed.
Reports suggest that the bivalent mRNA-1273.214 vaccine based on the Wuhan-Hu-1 and Omicron BA.1 spike mRNA has a slightly higher rate of adverse reactions. Moreover, no evidence of adverse reactions after bivalent COVID-19 vaccination is available due to approval without additional clinical studies.
 Study: Bivalent BNT162b2mRNA original/Omicron BA.4-5 booster vaccination: adverse reactions and inability to work compared to the monovalent COVID-19 booster. Image Credit: Akash Sain / Shutterstock
Study: Bivalent BNT162b2mRNA original/Omicron BA.4-5 booster vaccination: adverse reactions and inability to work compared to the monovalent COVID-19 booster. Image Credit: Akash Sain / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study and findings
In the present study, researchers in Germany and the United Kingdom evaluated adverse reactions, pro re nata (PRN) medication intake, and the ability to work after the second booster vaccination (fourth dose) among healthcare workers (HCWs). All participants had been previously administered European Medicines Agency (EMA)-approved primary COVID-19 immunization, followed by subsequent mRNA vaccine-based booster dose.
The second booster vaccine was either the monovalent BNT162b2 vaccine or the bivalent BNT162b2 vaccine with spike mRNA of wildtype and Omicron BA.4/5 variant. Participants who received a different vaccine as the second booster dose and those who received a concurrent influenza vaccination were excluded from the study.
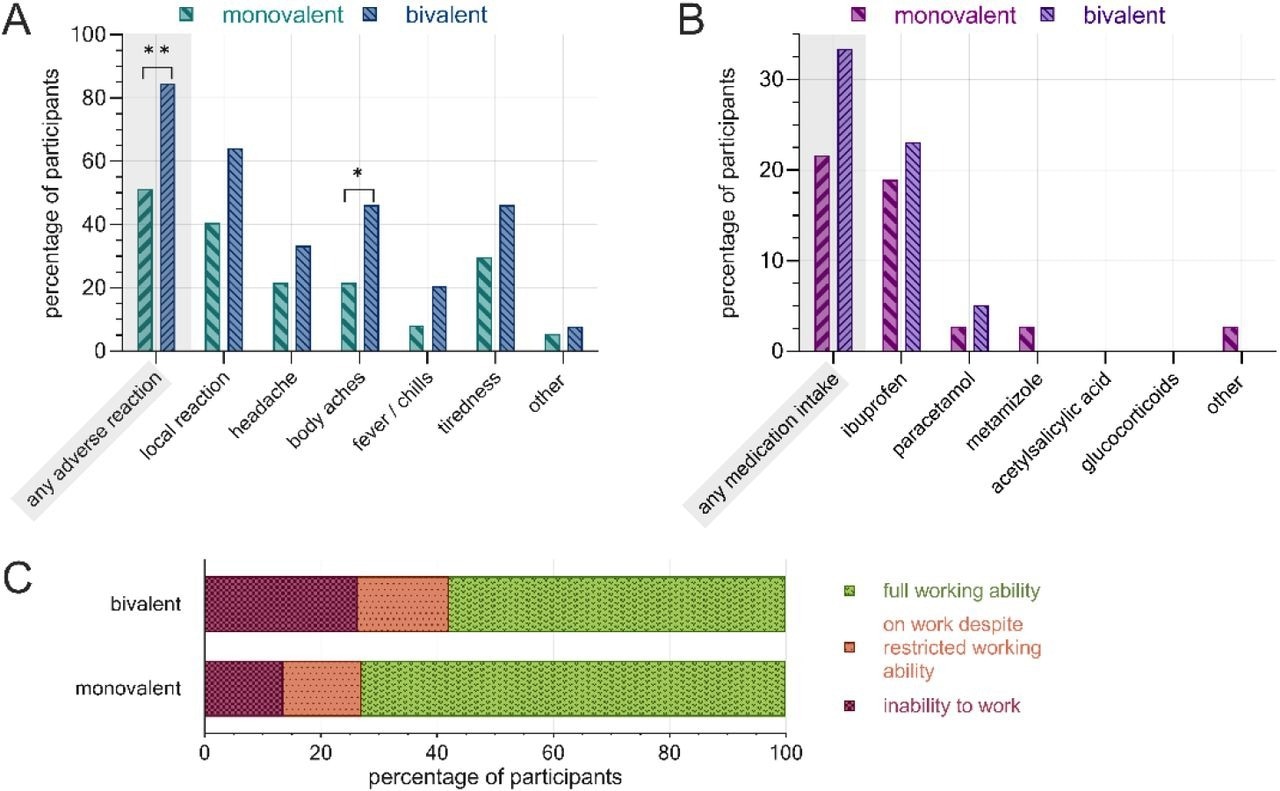 Post-vaccination adverse reactions, PRN medication and inability to work following the second COVID-19 booster administration, separated by vaccine. A) rate of adverse reactions by subcategory, B) rate of PRN medication, C) work ability restrictions. Monovalent: BNT162b2mRNA (n=37), bivalent: BNT162b2mRNA original/Omicron BA.4-5 (n=39). **: p<0.01, *: p<0.05.
Post-vaccination adverse reactions, PRN medication and inability to work following the second COVID-19 booster administration, separated by vaccine. A) rate of adverse reactions by subcategory, B) rate of PRN medication, C) work ability restrictions. Monovalent: BNT162b2mRNA (n=37), bivalent: BNT162b2mRNA original/Omicron BA.4-5 (n=39). **: p<0.01, *: p<0.05.
Data on adverse reactions, sociodemographic factors, PRN medication, and the ability to work were obtained by a questionary using Research Electronic Data Capture (REDCap) tool. In addition, the null hypothesis was tested using the Mann-Whitney U and Fisher’s exact tests. Seventy-six HCWs received the second COVID-19 booster from August 13, 2021, to October 14, 2022.
Thirty-seven HCWs received the monovalent BNT162b2 vaccine, and 39 received the bivalent vaccine (wildtype/Omicron BA.4/5). Most HCWs (80%) were female; the median age of female and male HCWs was 47 and 51, respectively. The rate of adverse reactions following the second booster administration was significantly higher among HCWs immunized with the bivalent vaccine (84%) than those receiving the monovalent vaccine (51%).
Specifically, the rates of headache, body aches, tiredness, fever, chills, and local reactions were significantly higher in HCWs receiving the bivalent vaccine. Bivalent vaccine-administered HCWs reported a more frequent PRN medication use and had elevated rates of workability restrictions than monovalent vaccine-administered restrictions.
Conclusions
The researchers observed that HCWs receiving the bivalent BNT162b2 wildtype/Omicron BA.4/5 vaccine as the second booster shot showed a higher prevalence of adverse reactions than monovalent vaccine-boosted HCWs. Notably, the interval between the first and second booster administration was 193 days for monovalent vaccine recipients and 322 days for bivalent vaccine recipients.
Furthermore, HCWs reported increased PRN medication intake and inability to work following bivalent booster dose administration. The study’s limitations include its retrospective questionnaire-based design and the lack of blinding and randomization. Overall, these findings may help inform clinical decisions regarding monovalent and bivalent vaccination.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Wagenhäuser I, Reusch J, Gabel A, et al. Bivalent BNT162b2mRNA Original/Omicron BA.4-5 Booster Vaccination: Adverse Reactions and Inability to Work Compared to the Monovalent COVID-19 Booster. medRxiv, 2022, DOI: 10.1101/2022.11.07.22281982, https://www.medrxiv.org/content/10.1101/2022.11.07.22281982v1
- Peer reviewed and published scientific report.
Wagenhäuser, Isabell, Julia Reusch, Alexander Gabel, Lukas B. Krone, Oliver Kurzai, Nils Petri, and Manuel Krone. 2023. “Bivalent BNT162b2mRNA Original/Omicron BA.4-5 Booster Vaccination: Adverse Reactions and Inability to Work Compared to the Monovalent COVID-19 Booster.” Clinical Microbiology and Infection, January. https://doi.org/10.1016/j.cmi.2023.01.008, https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(23)00030-7/fulltext