Why is HbA1c testing important? What is Boronate Fluorescence Quenching Technology and how does it measure the level of glycated haemoglobin (HbA1c)?
Glycated hemoglobin (HbA1c) is well recognized as a reliable measure for glycemic control. The role of HbA1c testing in the management of patients with diabetes has been well established for several decades. HbA1c levels reflect the average circulating glucose concentration over the lifespan of red blood cells which is between two to three months.
The glycated hemoglobin molecule in blood is highly stable once it has formed after exposure to glucose, since it binds irreversibly to a specific part of hemoglobin in red blood cells.
Therefore, use of HbA1c can offer greater clinical information than a single glucose measurement taken at a particular time. Furthermore, there is evidence that HbA1c is a good predictor of an individual’s risk of developing long-term complications of diabetes, e.g. cardiovascular disease.
Based on well documented boronate affinity for glycated hemoglobin, Boronate Fluorescence Quenching Technology (BQFT) has similar performance to the boronate affinity chromatography systems used in reference laboratories.
As our reagent comes into contact with HbA1c in a blood sample the fluorescence is reduced/quenched. The rate of this quenching is directly related to the amount of HbA1c available in the blood.
Boronate Fluorescence Quenching Technology used to measure HbA1c in Quo-Lab
The Quo-Test and Quo-Lab systems employing the BFQT have the distinct advantage of not being affected by endogenous interferences like hemoglobin variants and labile glycated hemoglobin. As BFQT does not require chromatographic separation, the methodology allows for fast, simple and accurate point of care HbA1c measurement.
Please describe the Quo Test and Quo Lab solutions for diabetes POC testing. What advantages do they give healthcare professionals when diagnosing diabetes?
The Quo-Test and Quo-Lab offer lab-quality results from just 4 µL of blood in 4 minutes. With traditional methods of detecting or monitoring diabetes you would either need to have an Oral Glucose Tolerance test (OGTT) or a fasting glucose test, both of which take a considerable amount of time and are susceptible to considerable error.

Image Credit: EKF Diagnostics
Even clinical methods of testing HbA1c can take a few days to get results back from the laboratory where as POCT methods allow results to be delivered to the patient right there and then whilst sat in front of the consulting physician.
Evidence suggests that this level of engagement has a significant impact upon how those with diabetes understand and act upon their results. POCT has been proven to drive down average HbA1c levels over a shorter amount of time than when results are delivered days later, when the patient is likely to be less engaged with the management of their health.
What advantages does the Quo Connectivity solution bring to the Quo Test and Quo Lab analyzers?
Previously it had only been possible to add a patient ID and user ID to each test result through the use of a barcode scanner, although this process kept the routine simple, transparent and suitable for a global market we found that many hospitals and clinics needed something more.
It is now possible to record an increased amount of demographic information including full patient name, date of birth and patient ID numbers using either the standard product barcode scanner or the new keyboard accessory.
This additional demographic information enables the patient and their results to be linked and traced right throughout the healthcare system. There is also space for additional commentary associated with each result so that other symptoms and observations can be recorded. As healthcare departments and facilities become more connected, it becomes more important to make linking different patient record systems easy.
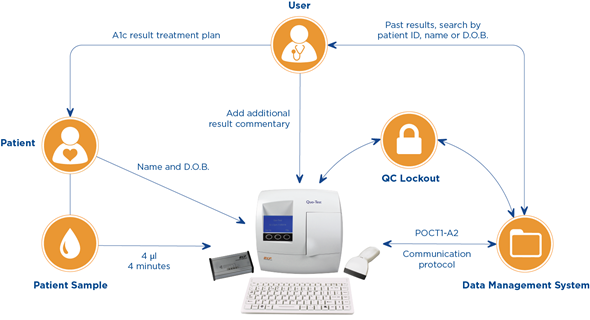
Image Credit: EKF Diagnostics
How does the Quo connectivity solution connect to a central data management networks? What constraints are there on these connections?
The healthcare industry has not yet been able to agree on a harmonized and approved standard for connectivity communication, it is a complicated process with many factors of security and patient confidentiality to consider.
The closest they have come to date has been the POCT-1A protocol, which itself is in its second iteration and still has not been fully approved. The Quo connectivity solution uses this POCT-1A2 communication protocol and the in-house designed Connector Interface Box to create bidirectional communication between a multitude of Lab Information Management Systems (LIMS) and the Quo systems.
Unlike many closed proprietary data management systems, our solution is open, allowing for easy and simple connection to whatever system the clinic is currently using.
What enhanced security protocols does the Quo connectivity bring to the Quo range? What advantages are there to restricting access to trained personnel?
Operator IDs can now be added to each test result, significantly improving the traceability and security applied to every HbA1c reading. A controlled list of trained operators assures that only those with sufficient competency have access to the system to start taking measurements.
This new feature takes away the risk that an untrained operator can access the system or even review patient results unless they have the proper and correct security level.
How important are quality controls of HbA1c tests? What quality controls are enforced with the Quo Connectivity solution?
Quality control is always an important element to any diagnostic test, even more so when we are talking about POCT systems used outside of the lab. Enhanced quality control is now possible with multiple user-defined QC lockout options available to POCT coordinators, ensuring that tests can only be run according to whatever quality assurance procedure is required.
Even more traceability is possible as consumables and quality controls can now be added to an approval list meaning only those products that have passed incoming inspection are used.
What does the future hold for POC diabetes testing and the Quo range?
As the demand for connected systems with better accuracy and precision becomes greater we will start to see a change in the type of products clinicians utilize.
Currently Blood Glucose Meters (BGMs) are used in hospital wards across the globe, this is unacceptable. These devices are designed for home–use, to aid in diabetes management; they do not have the accuracy, precision or sophistication required to make informed clinical decisions.
The FDA highlighted this recently in their draft guidelines which suggested that clinically suitable products should have 10% accuracy as standard rather than the 20% that was currently on offer.
The situation is not quite the same when it comes to HbA1c, it is not helpful to test HbA1c at home and we are likely already close to the limits of detection when it comes to biological variability but there are forward steps that can be made.
Open yet secure connectivity is the first step in that direction, the next is likely taking that data management to the next level by making it available on the cloud and accessible to any healthcare system regardless of whether they are your usual provider or even in the same country.
The globalization of personalized healthcare is a lot closer than we think but still not close enough to touch…yet.
Finally, when will the connectivity solution from EKF Diagnostics be ready?
The Quo-Test and Quo-Lab connectivity solution is being previewed at Medica on the EKF Diagnostics stand (Hall 3 Stand no. C70). The company aim to officially launch the product in Q1 2017.
Where can readers find more information?
For more information on the Quo products please visit our EKF Diagnostics targeted microsite www.hba1c-test.com.
About Gavin Jones
 Gavin Jones has overseen the marketing and development of EKF Diagnostics’ diabetes portfolio for the past four years. Gavin attended Liverpool John Moores University where he attained a Bachelor of Science in Forensic and Biomolecular Sciences.
Gavin Jones has overseen the marketing and development of EKF Diagnostics’ diabetes portfolio for the past four years. Gavin attended Liverpool John Moores University where he attained a Bachelor of Science in Forensic and Biomolecular Sciences.
He has worked in multiple scientific fields from forensic toxicology for the Home Office through to analytical chemistry in the pharmaceutical and finally settling now in the life sciences focused on development of POCT medical devices.