Researchers in Milan, Italy, have identified the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the heart tissue of deceased coronavirus disease 2019 (COVID-19) patients who had died from respiratory failure, without showing any signs of cardiac involvement.
The virus was present in the cardiomyocytes of all six patients, and active viral transcription of RNA for the viral spike protein had occurred. The spike protein is the surface structure SARS-CoV-2 uses to bind to and access host cells.
The injury to cardiomyocytes varied, from the absence of cell death and no subcellular alterations to structural abnormalities and intracellular edema.
Gaetano Pietro Bulfamante (University of Milan) and colleagues say the findings indicate that guidelines for monitoring COVID-19 survivors once they have been discharged from hospital need to be revised.
The long-term cardiovascular outcomes could be similar to those seen in survivors of the 2002 to 2003 SARS-CoV-1 outbreak, where 40% of recovered individuals developed cardiovascular abnormalities over a 12-year follow-up period, the team warns.
A pre-print version of the paper is available on the server medRxiv* while the article undergoes peer review.
COVID-19 can involve the cardiovascular system
As the SARS-CoV-2 pandemic continues to sweep the globe, the virus is continuing to infect hundreds of thousands of people every day and COVID-19 mortality is still increasing worldwide.
Aside from the respiratory illness the virus causes, studies have now shown that several other organs may be involved, including the cardiovascular system.
Between 20 and 40% of hospitalized patients experience cardiac symptoms, ranging from chest pain to arrhythmia and cardiogenic shock.
Furthermore, up to 7% of COVID-related deaths are linked to myocarditis, say Bulfamante, and team.
However, to date, it is still not clear whether cardiovascular involvement is due to cells being directly damaged by the virus or whether it is secondary to the involvement of an overreactive immune response. Hypoxia-induced by acute respiratory distress syndrome (ARDS) might also lead to myocardial injury.
On the other hand, SARS-CoV-2 has been identified in patients’ vascular endothelial cells and the cardiac macrophages of patients with myocarditis. Furthermore, viral RNA has previously been identified in whole heart tissue, irrespective of cardiac phenotype, say the researchers.
“Nonetheless, immunohistochemistry for viral antigen detection in cardiomyocytes has never been shown,” writes the team.
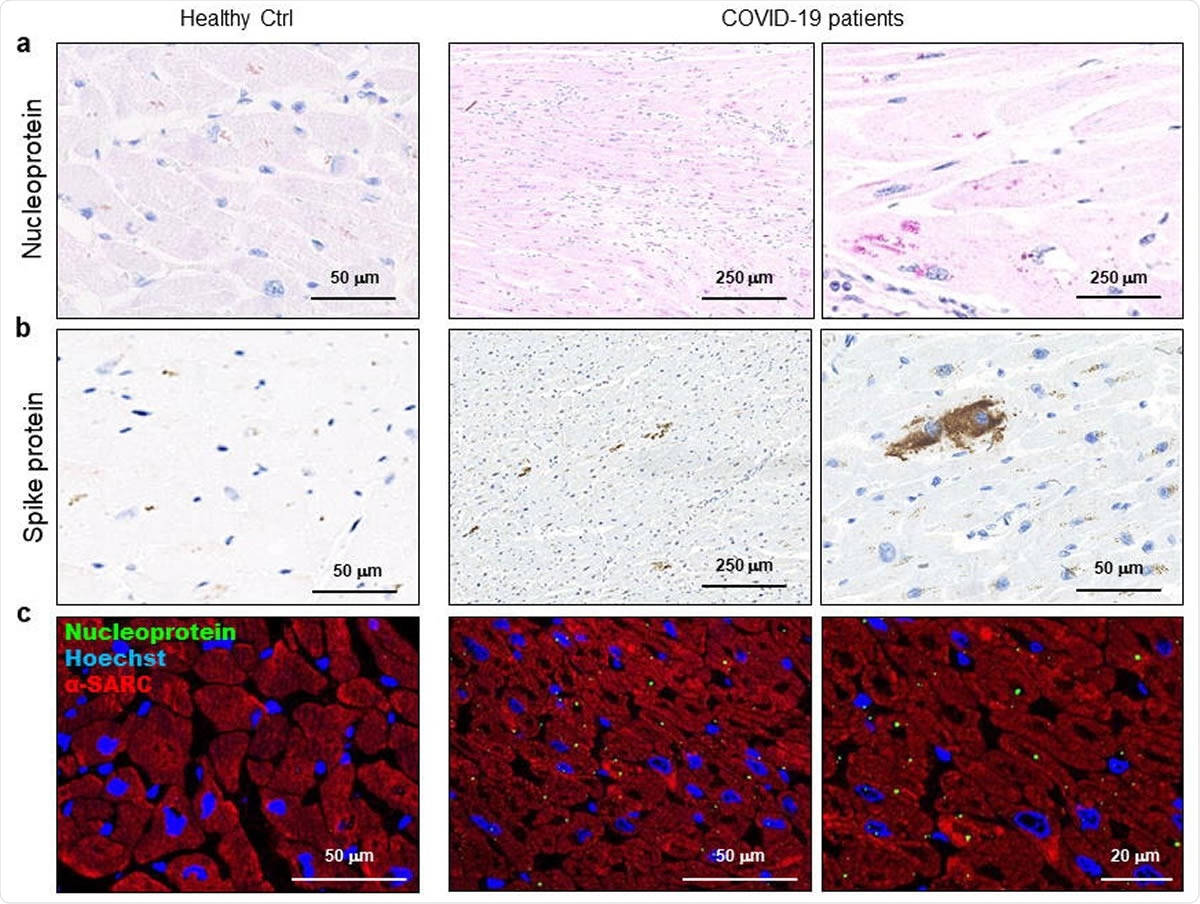
SARS-CoV-2 proteins are detectable in cardiomyocytes of COVID-19 patients. a-b) Representative images of immunohistochemistry assays on 3μm-slides of formalin-fixed paraffin-embedded left ventricle specimens from healthy control (Healthy Ctrl, left panels) and COVID-19 patients (COVID-19, central and right panels) for SARS-CoV-2 nucleoprotein (a, in red) and spike protein (b, in brown). c) Representative images of immunofluorescence assay on 3μm-slides of formalin-fixed paraffin-embedded left ventricle specimens from healthy control (Healthy Ctrl, left panels) and COVID-19 patients (COVID-19, central and right panels) for SARS-CoV-2 nucleoprotein (green) and sarcomeric α-actin (α-SARC, red). Nuclei have been stained by Hoechst (blue). Magnification as scale bars.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What did the study involve?
The researchers tested for the presence of SARS-CoV-2 in the heart tissue of six patients (aged 54 to 69 years) with COVID-19 who had died from respiratory failure, without showing signs of cardiac involvement.
Using chip-based digital PCR, the team detected SARS-CoV-2 viral RNA in the cardiomyocytes of heart tissue samples taken from all six patients. Using Western blot, immunohistochemistry and immunofluorescence techniques, they also identified viral spike protein in the cardiomyocytes and electron microscopy revealed the presence of full virus particles.
Interestingly, the researchers found that the expression of the spike protein varied between patients, which led them to the hypothesis that the viral genome was being actively transcribed within the cardiomyocytes.
To test for the presence of SARS-CoV-2 as a transcriptionally active virus in the cells, the team performed an RNAScope assay using two probes that recognize spike protein sense and antisense RNA, to enable distinction between the viral RNA genome and its transcript. Signals were obtained with both probes for all patient samples, but the most abundant staining was observed for the probe that detected the actively transcribed virus.
The researchers say this is the first study to identify the localization of SARS-CoV-2 RNA in cardiomyocytes and the active viral transcription of both sense and antisense RNA for the viral spike protein.
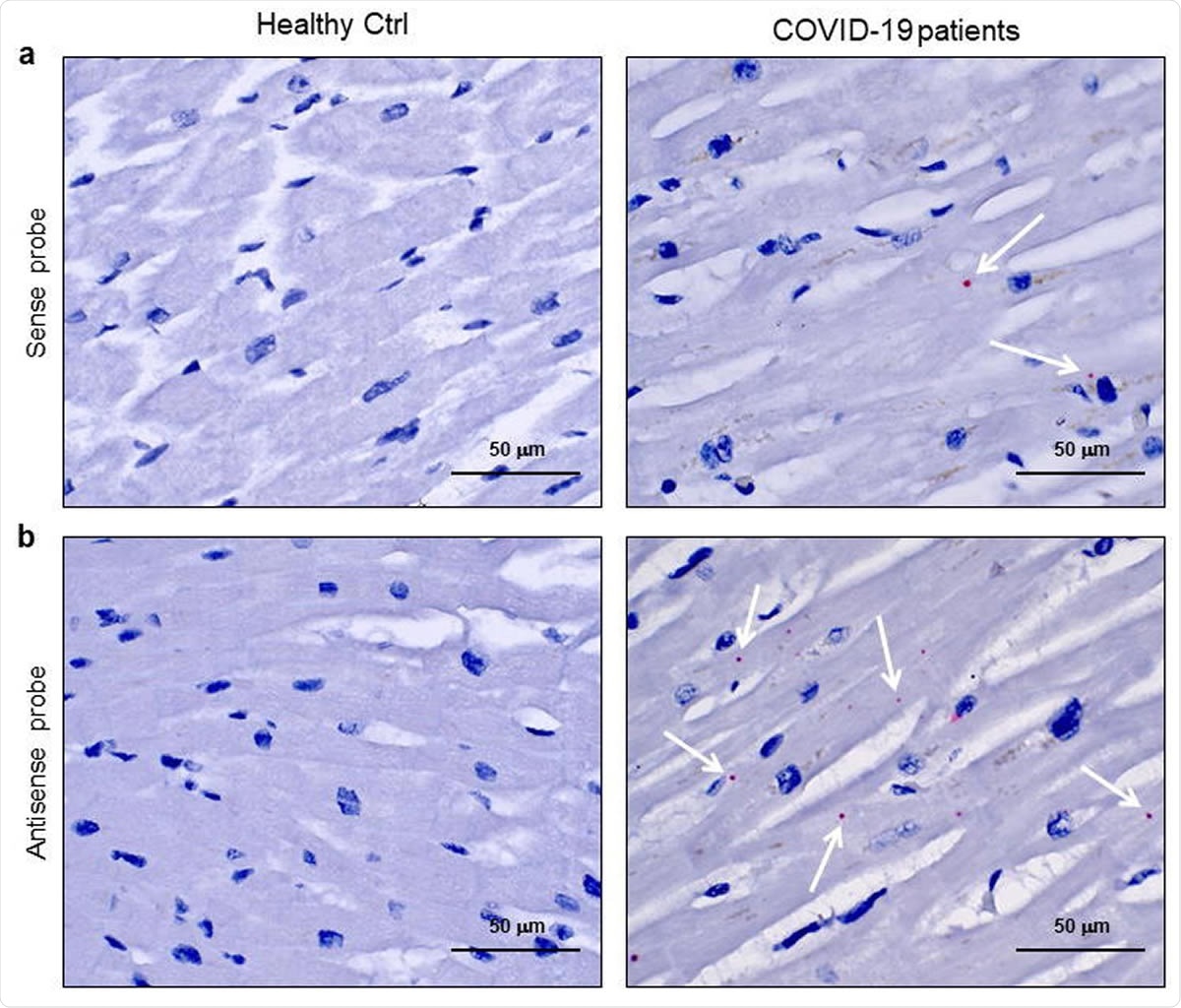
SARS-CoV-2 sense and antisense RNA are localized into cardiomyocytes of COVID-19 patients. a-b) Representative images of spatially resolved viral RNA detection by RNAScope assay on 3μmslides of formalin-fixed paraffin-embedded left ventricle specimens from healthy control (Healthy Ctrl, left panels) and COVID-19 patients (COVID-19, central and right panels) for SARS-CoV-2 sense (a) and antisense (b) probes for spike protein RNA sequences. Fast Red dots indicate viral RNA presence (white arrows). Nuclei have been counterstained with haematoxylin. Magnification as scale bars.
“A precocious subclinical sign of cell damage”
The pattern of cardiomyocyte injury varied. Cells containing the virus did not appear to undergo apoptosis but did exhibit intracellular alterations, including increased cell volume and intracellular edema.
While some cardiomyocytes containing SARS-CoV-2 appeared normal, others showed excessive intracellular area between sarcolemma and sarcomere structure, which the authors say may explain the intracellular edema observed.
“Several cardiomyocytes showed an altered cell structure, with increased cell area and intracellular edema, suggesting a cardiomyocyte swelling,” writes the team. “This microscopic event may be interpreted as a precocious subclinical sign of cell damage, potentially leading to myocardial interstitial edema formation.”
Post-discharge surveillance guidelines need revising
The researchers say the study has shown that cardiac tissue, including the contractile cell compartment, can be infected by SARS-CoV-2 and that initially, this may cause microscopic cardiomyocyte alterations that do not lead to clinically-relevant macroscopic cardiac damage.
“Our crucial study specifically suggests the need to redefine post-discharge surveillance guidelines for surviving COVID-19 patients’ follow-up even in the absence of overt cardiac phenotype,” say Bulfamante and colleagues.
“The long-term cardiovascular outcome of COVID-19 patients may mirror SARS patients, who developed cardiovascular abnormalities in 40% of recovered cases in a 12-years-long follow-up period,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Evidence of SARS-CoV-2 transcriptional activity in cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. medRxiv 2020. doi: https://www.medrxiv.org/content/10.1101/2020.08.24.20170175v1
- Peer reviewed and published scientific report.
Bulfamante, Gaetano Pietro, Gianluca Lorenzo Perrucci, Monica Falleni, Elena Sommariva, Delfina Tosi, Carla Martinelli, Paola Songia, Paolo Poggio, Stefano Carugo, and Giulio Pompilio. 2020. “Evidence of SARS-CoV-2 Transcriptional Activity in Cardiomyocytes of COVID-19 Patients without Clinical Signs of Cardiac Involvement.” Biomedicines 8 (12): 626. https://doi.org/10.3390/biomedicines8120626. https://www.mdpi.com/2227-9059/8/12/626.