A team of scientists from the University of Maryland School of Medicine in the United States has recently evaluated the immunological impacts of single-dose coronavirus disease 2019 (COVID-19) vaccination on healthcare workers.
The findings reveal that healthcare workers previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) develop significantly higher antibody titers in response to a single dose of COVID-19 vaccine, as compared to healthcare workers without prior SARS-CoV-s infection. The study is currently available on the medRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Since its emergence in December 2019 in China, more than 103 million confirmed COVID-19 cases, including 2.25 million deaths, were reported to the World Health Organization (WHO). To tackle the rapid spread of SARS-CoV-2, the entire scientific community has made a significant effort to develop specific therapeutics and vaccines. Currently, several potential COVID-19 vaccines with good safety and efficacy levels are rolling out in many countries. The primary aim of such vaccination programs is to immunize and protect frontline workers, such as healthcare workers and at-risk individuals.
However, because of the deficiencies in vaccine production and distribution, some countries have started considering suboptimal vaccine doses (half or single dose) to maximize the coverage. Although clinical studies have shown that people with prior SARS-CoV-2 infection develop adaptive immunity that can protect them from reinfection for at least six months, it is still not known whether a suboptimal vaccine dose can induce desired immune responses in these individuals.
In the current study, the scientists have examined the antibody responses induced by a single dose of COVID-19 vaccine in healthcare workers with or without prior SARS-CoV-2 infection.
Study design
The healthcare workers who participated in the study were categorized into three groups: anti- SARS-CoV-2 IgG positive participants with a history of symptomatic COVID-19; anti-SARS-CoV-2 IgG positive with a history of asymptomatic COVID-19; and IgG negative participants. The immunological impacts of a single dose of Pfizer-BioNTech or Moderna vaccine were tested. The levels of SARS-CoV-2 spike trimer-specific IgG antibodies (antibody titers) were measure in plasma samples at baseline (day 0) and after 7, 10, and 14 days of vaccination.
Important observations
A total of 59 healthcare workers participated in the study. The findings revealed that in response to a single vaccine dose, the participants with prior COVID-19 exposure developed significantly higher spike-binding antibody titers than those without any history of SARS-CoV-2 infection. Similarly, significantly higher levels of virus-neutralizing antibodies were observed in participants with prior SARS-CoV-2 infection than those without infection.
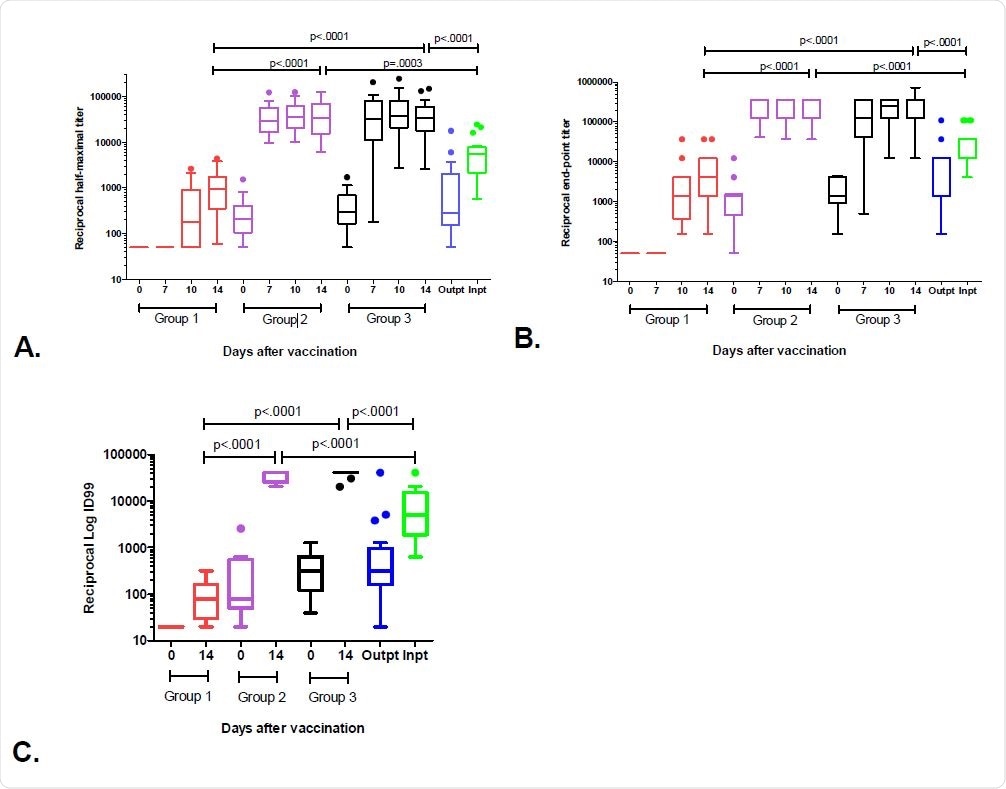
Anti-SARS-CoV-2 antibody responses after a single dose of vaccination. After COVID-19 vaccination, plasma was drawn at 0, 7, 10, and 14 days and IgG binding titers against spike measured by ELISA and live virus neutralization at Day 0 and 14 measured. Single time point was also measured among COVID-19 outpatients or inpatients whose blood was drawn during peak antibody production (1-2 months after the onset of symptoms). A) IgG spike trimer half-maximal titers. By 7 days and continuing to 14 days post-vaccination HCW with prior COVID-19 who received a single vaccination developed higher peak IgG titers than HCW without COVID and COVID-19 infected patients. Half-maximal binding titers represent the dilution of plasma that achieves 50% of the maximal binding of a known control that reaches saturation. B) IgG spike trimer end-point titers. By 7 days and continuing to 14 days post-vaccination HCW with prior COVID-19 who received a single vaccination developed higher peak IgG titers than HCW without COVID and COVID-19 infected patients. End-point binding titers represent the highest dilution of plasma that gives a reading above the statistically defined cutoff. C) Live virus neutralization. Serial dilutions of plasma were incubated for one hour with 100TCID50 of WA-1 strain SARS-CoV-2. This admixture was added to Vero cells, and the cytopathic effect was assayed using live/dead staining at 72 hours. At 14 days, HCW with prior COVID-19 who received a single vaccination developed higher neutralization titers than HCW without COVID and COVID-19 infected patients. Neutralization values greater than 40,960 were reported as 40,960, accounting for small box size and single line seen in Groups 2 and 3. ID99 defined as the highest dilution at which 99% of cells were protected. Group 1= SARS-CoV-2 IgG negative healthcare worker (HCW). Group 2= asymptomatic SARSCoV- 2 IgG positive HCW. Group 3= symptomatic SARS-CoV-2 IgG positive HCW. Box plots represent 25% to 75% percentile, with individual dots representing outliers using Tukey’s method (1.5 x IQR).
Study significance
The study highlights that healthcare workers with prior SARS-CoV-2 infection can develop significant levels of secondary antibody responses in response to a single dose of mRNA vaccine containing SARS-CoV-2 spike protein as a vaccine antigen.
In previously infected healthcare workers, both spike-binding and neutralizing antibody titers started rapidly increasing by day 7 of vaccination and achieved a higher level at day 14 of vaccination. Importantly, a comparable level of binding and functional antibody titers has been observed among healthcare workers with prior symptomatic or prior asymptomatic infection. Moreover, the findings reveal that the antibody titer developed in response to a single vaccine dose is higher than that developed by natural infection and similar to that developed by two doses of these mRNA vaccines. Activation of memory B cells developed in response to prior SARS-CoV-2 infection might be the reason for such heightened secondary antibody responses.
The study suggests that a single dose of vaccine can trigger the activation of an already existing pool of memory antibodies (recall response) in people with previous SARS-CoV-2 infection. According to the scientists, such a single-dose vaccination regimen can be adopted in vaccine shortage times to maximize the coverage.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources