COVID-19 vaccines remain the best tool in the pandemic toolbox against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). But they are not perfect; reports have surfaced of mild to severe side effects after vaccination. A new study from researchers in the Lampe & Company GmbH & Co. KG compared the severity of adverse events from each coronavirus vaccine.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Compared to COVID-19 infection and the risk of long COVID, vaccine side-effects are temporary and are unlikely to cause hospitalization. But their findings suggest every vaccine carries a common risk of fatigue, headache, and muscle weakness. Additionally, people 18 to 55 years old were more likely to experience vaccine side-effects than people over the age of 55.
Understanding the side effects of each vaccine may help in shaping the messaging surrounding vaccine safety, help in planning for future vaccination campaigns, and inform the public on their decision to get vaccinated.
“The multidimensional assessment of published vaccine data presented here may serve as a basis for a public awareness campaign to combat vaccine hesitancy by determining and explaining the phenomena which the public perceives intuitively,” concluded the researchers.
The study “An objective systematic comparison of the most common adverse events of COVID-19 vaccines” is published as a preprint on the medRxiv* server.
How they did it
The researchers performed a systemic search of 10 journal articles and 2 regulatory documents available from May 31, 2021, that researched adverse events after COVID-19 vaccination. Articles used by the U.S. Food & Drug Administration and the European Medical Agency to approve the vaccines were also taken into consideration.
The vaccines focused on for this study were the Pfizer-BioNTech vaccine, the Moderna vaccine, and the AstraZeneca/Oxford vaccine.
In total, there were 66 different study arms with relevant information on vaccine safety.
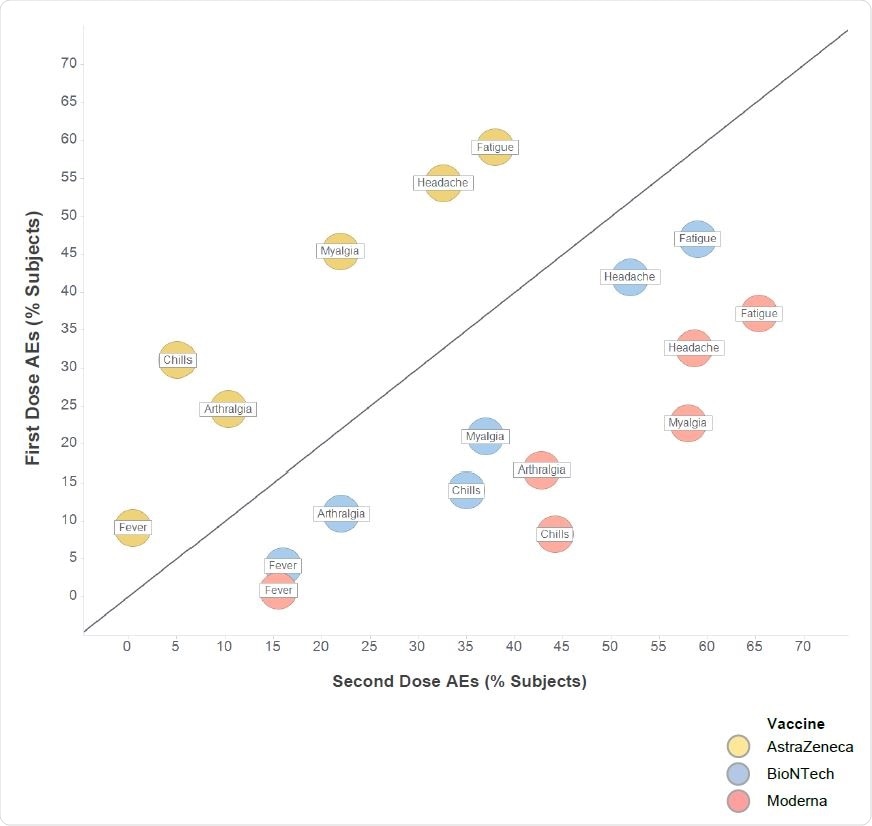
Most common systemic adverse events occurring after the first and second doses of anti293 SARS-CoV-2 vaccines. The highest frequencies for each adverse event occurring with each vaccine are shown. For BioNTech only young/middle-aged subjects (18–65 years) are included; no data were available for the overall population. Sources: AstraZeneca (AZ): EMA Assessment Report (2021); BioNTech (BNT): Polack et al. (2020); Moderna (MOD): Baden et al. (2020).
COVID-19 vaccines show a good safety profile with mild adverse events
All three COVID-19 vaccines shared the following mild side-effects: fatigue, headache, and muscle weakness. But overall, all were well-tolerated and temporary.
Side-effects were most reported after the first AstraZeneca vaccine dose. In contrast, both mRNA vaccines showed the most reports for adverse events after the second dose.
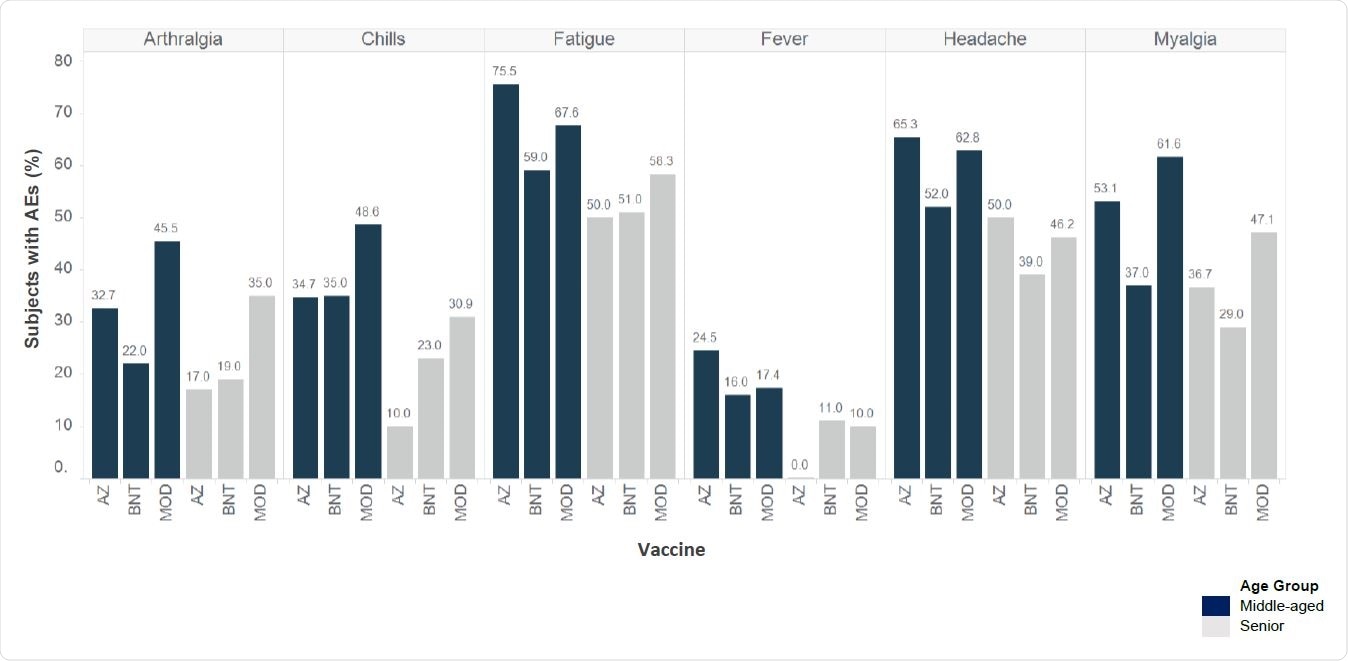
Overall frequency of systemic adverse events by age group observed with the anti-SARS-CoV-2 vaccines. Shown here are the highest observed frequencies of each adverse event, independent of dose (first or second). *Maximum age for middle-aged subjects and minimum age for senior subjects varies from 55–65 years. Sources: AstraZeneca (AZ): Ramasamy et al. (2020); BioNTech (BNT): Polack et al. (2020); Moderna (MOD): Baden et al. (2020)
The number of adverse events from the first AstraZeneca dose corresponded to the number of adverse events observed with the second mRNA dose.
Vice versa, the number of adverse events from the second AstraZeneca dose was similar to the number of adverse events from the first mRNA doses.
“Overall, regardless of dose, the frequency of each AE was very similar across vaccines and did not show a tendency towards one vaccine over another, independent of the used platform (non-replicating viral vector or mRNA),” wrote the research team.
People between the ages of 18 to 55 reported more vaccine side effects than people over the age of 55. This observation was seen amongst all three coronavirus vaccines.
Because the risk of blood clots and thrombosis is rare after vaccination, the researchers did not find reports of it in any of the clinical trials.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.