Pregnant women appear to be at a greater risk of severe coronavirus disease 2019 (COVID-19), as identified by increased reports of intensive care unit (ICU) admission, mechanical ventilation, and death in this patient population. Unfortunately, the COVID-19 pandemic has worsened existing perinatal inequities among communities that already faced higher rates of maternal mortality and morbidity, along with poor infant outcomes.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Although pregnant women were not included in the first clinical trials of COVID-19 vaccines, evidence suggests that vaccination reduces the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnant women. Further, maternal immunoglobulin G (IgG) antibodies induced after natural infection and vaccination can be detected in the umbilical cord of newborns at birth. These antibodies might help to protect newborns from SARS-CoV-2 infections during their early life.
The BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines, which were developed against the SARS-CoV-2 spike protein, were found to show greater than 90% efficacy against COVID-19 for at least four months. However, the emergence of several SARS-CoV-2 variants of concern (VOCs) has altered the transmissibility and sensitivity to neutralizing antibodies (nAbs).
SARS-CoV-2 VOCs include the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) variants. Among them, the Delta variant has been reported to be highly contagious and is associated with reduced neutralization in fully vaccinated individuals.
The SARS-CoV-2 Kappa (B.1.617.1) variant, which originated from the same lineage as as the Delta variant, has been categorized as a variant under monitoring (VUM). A new variant Mu (B.1.621) is more resistant to the BNT162b2 vaccine or sera from convalescent patients as compared to the wild-type strain.
Previous studies indicate that the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein consists of several conformational neutralizing epitopes. These are the sites where single amino acid mutations such as L452 (Kappa and Delta), N501 (Alpha and Delta), and E484 (Kappa and Mu) lead to altered transmission and antibody-mediated neutralization. The Delta variant also contains a specific T478K mutation that improves its interaction with the human angiotensin-converting enzyme (ACE-2) receptor.
Several studies have indicated that maternal IgG production and maternal-fetal transfer can be influenced by antibody glycosylation profiles, time of exposure, or fetal sex. However, the neutralizing activity against SARS-CoV-2 variants during pregnancy, as well as transplacental transfer efficacy, remains unstudied.
About the study
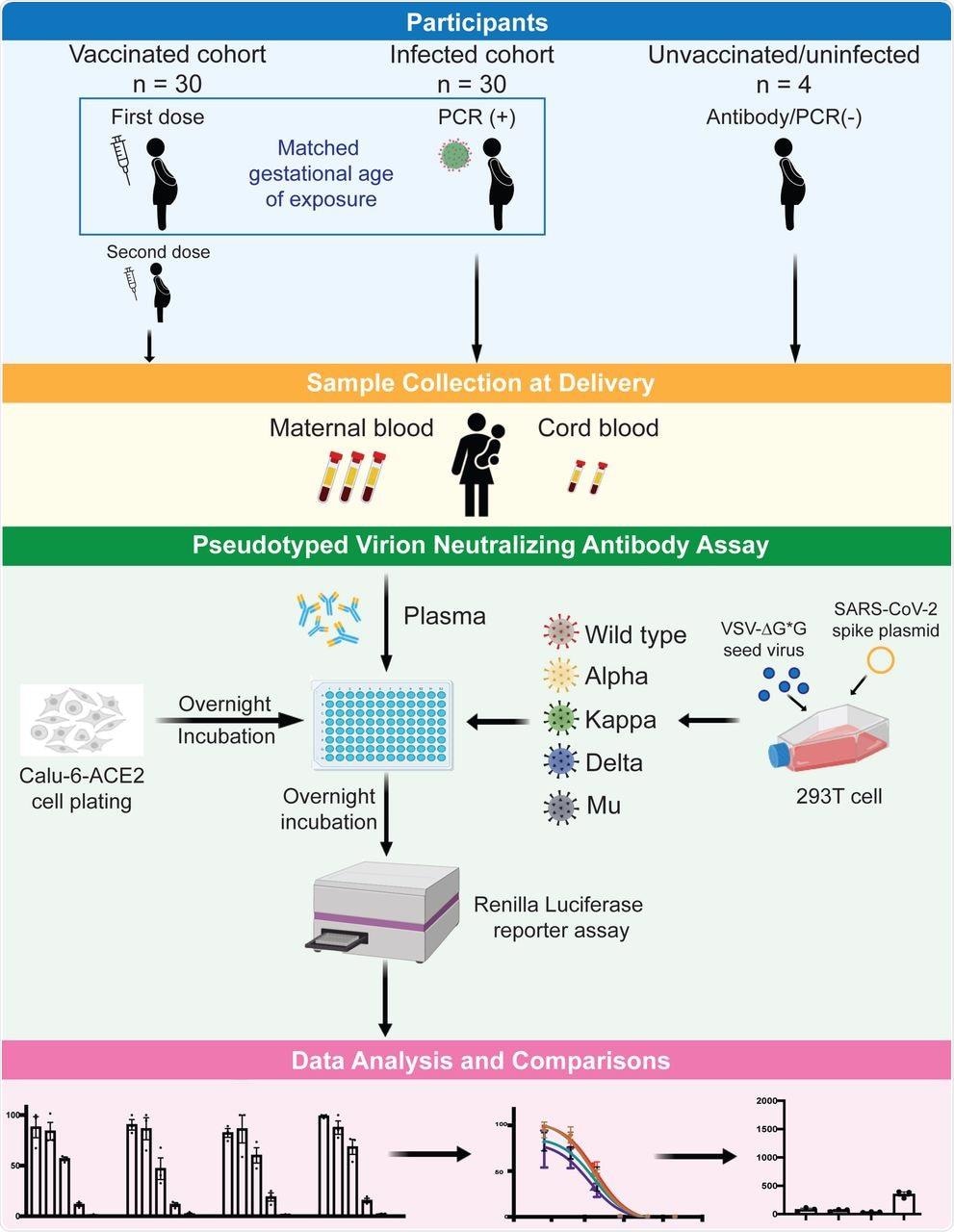
A new study published on the preprint server medRxiv* assessed nAb activities against five strains of SARS-CoV-2 including the wild-type (WT) (Wuhan-Hu-1), Alpha, Kappa, Delta, and Mu variants in paired maternal and infant cord blood plasma samples that were collected during the time of delivery.
Samples were also collected from both vaccinated and infected pregnant women to compare the nAbs generated by vaccine versus natural infection, their efficacy against the new variants, clinical correlates of nAb production, and impact of timing of vaccination or natural infection on neonatal and maternal protection.
The current study included pregnant individuals who had a positive SARS-CoV-2 test and individuals who were fully vaccinated with a messenger ribonucleic acid (mRNA)-based COVID-19 vaccine. Thirty individuals from each cohort were chosen and matched on the approximate gestational age of the first vaccine dose and the earliest confirmed COVID-19 test.
Infant cord blood and maternal blood were collected during delivery. Thereafter, plasma was isolated from the blood and stored for further analysis. Four individuals who were unvaccinated with no evidence of prior infection served as negative controls.
Pseudotyped virions were prepared by transfecting 293T cells with the spike plasmid followed by neutralization antibody assay using the pseudotyped virion. Finally, SARS-CoV-2 IgG antibodies were measured using the Pylon 3D automated immunoassay system.
Study findings
A total of 64 maternal-fetal dyads were identified in the study. Among the 30 vaccinated pregnant women, five had received the vaccine during the first trimester, 12 had received it during the second, and 13 had received it during the third trimester.
Among the 30 naturally infected pregnant women, five tested positive during the first trimester, 13 during the second, and 12 during the third. The maternal age was found to be higher in the vaccinated group as compared to the naturally infected group.
The delivery took place 91 days after the first dose of the vaccine for vaccinated mothers (30 dyads), whereas the delivery took place 92 days after the earliest positive test from infected mothers (30 dyads). Among the SARS-CoV-2 infected group, five were asymptomatic, 20 experienced mild disease, and three experienced severe disease.
Neutralization titers were found to be higher in cord blood from vaccinated mothers as compared to infected mothers. However, no difference was observed in the case of maternal blood.
No neutralizing activity was detected for uninfected maternal, unvaccinated, and infant dyad samples. The total neutralizing activity detected in maternal and cord blood samples collected during delivery increased with vaccination later in pregnancy.
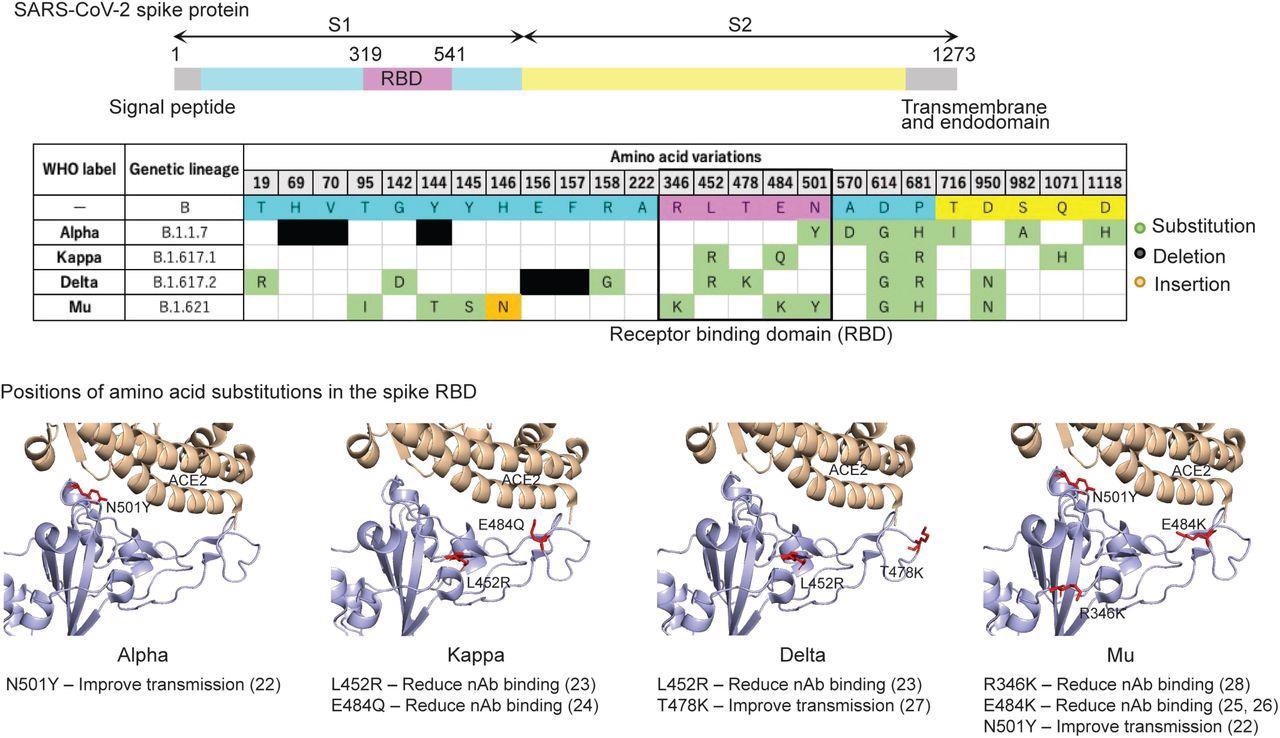 Positions of mutated amino acid residues in the Spike proteins from four variants. The residues L452R, T478K, R346K, E484K, and N501Y are labeled in red. The crystal structure of SARS-CoV-2 spike RBD and ACE2 (PDB: 6M0J) (64) was used as the template. All structure figures were generated with PyMol (65).
Positions of mutated amino acid residues in the Spike proteins from four variants. The residues L452R, T478K, R346K, E484K, and N501Y are labeled in red. The crystal structure of SARS-CoV-2 spike RBD and ACE2 (PDB: 6M0J) (64) was used as the template. All structure figures were generated with PyMol (65).
Vaccination was found to generate higher titers of nAb within the first few weeks in pregnant individuals that gradually waned. However, infection-elicited antibodies remained stable until delivery in both maternal and cord blood.
The results also indicated that for both the vaccinated and infected cohort, the Mu variant showed the highest resistance to nAb inhibition. The nAb activity of the Alpha variant was not significantly different from the WT variant for both groups. Although variable nAb response was observed against the different SARS-CoV-2 variants, neither trimester of exposure or vaccination/infection cohort had a role in the variability.
The transfer efficiency of maternal IgG antibodies was observed to increase during the first and second trimester for the vaccinated cohort, while no such increase was observed for the infected cohort. No significant difference was observed for the vaccinated cohort during the third trimester, while a reduction in transfer efficacy was observed during the third trimester for the infected cohort.
The maternal cord nAb transfer ratio (TR) was found to be less than one when mothers were vaccinated in the third trimester and less than 60 days before delivery, while it was more than one for greater than 60 days. The TR was found to be higher in the vaccinated cohort as compared to the infected cohort. Furthermore, when a stratified analysis based on SARS-CoV-2 strains was conducted, the TR for the Delta variant was found to be higher in the vaccinated cohort as compared to the infected cohort.
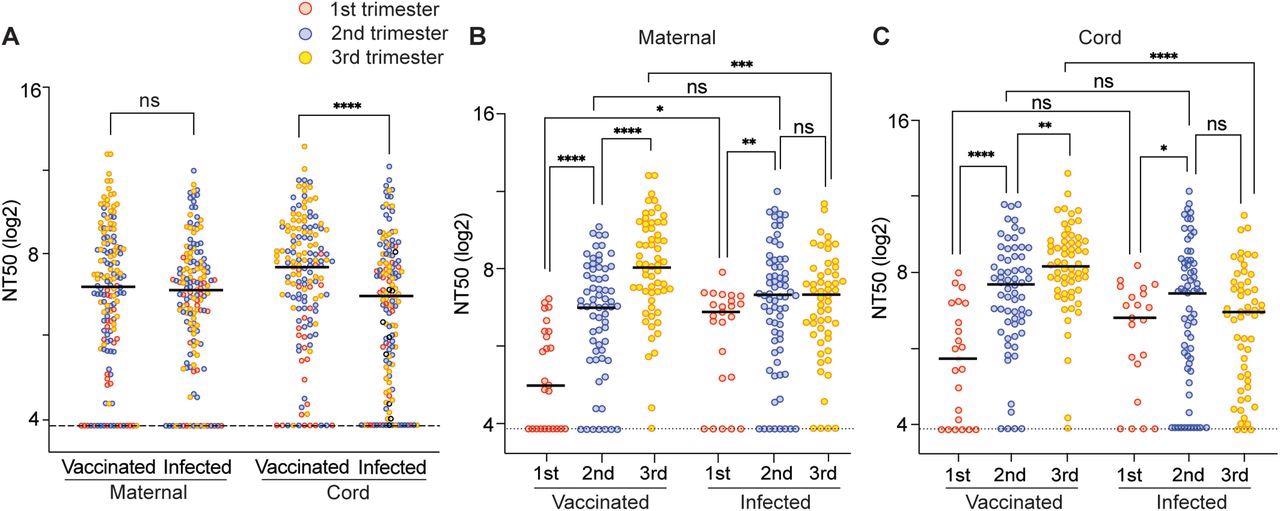 Neutralizing activity of maternal and cord plasma samples from vaccination cohort and infection cohort. (A) Comparison of the composite median NT50 titers against all examined strains between the vaccinated and infected group. The dot plots show NT50 values in maternal plasma and cord plasma. The dotted line indicates the cut-off threshold of this assay. (B, D) NT50 values of the maternal and cord blood were compared separately. Black bars represent the median of NT50 values. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant (Mann-Whitney test). The trimester of exposure is indicated by color (red, first trimester; blue, second trimester; yellow, third trimester).
Neutralizing activity of maternal and cord plasma samples from vaccination cohort and infection cohort. (A) Comparison of the composite median NT50 titers against all examined strains between the vaccinated and infected group. The dot plots show NT50 values in maternal plasma and cord plasma. The dotted line indicates the cut-off threshold of this assay. (B, D) NT50 values of the maternal and cord blood were compared separately. Black bars represent the median of NT50 values. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant (Mann-Whitney test). The trimester of exposure is indicated by color (red, first trimester; blue, second trimester; yellow, third trimester).
The nAb was found to be lower for male fetuses as compared to female fetuses during the first trimester; however, it was found to be higher during the second trimester. Moreover, individuals with a body mass index (BMI) of 25-29.9 were found to have higher nAb in both maternal and cord blood as compared to those with a BMI of less than 25 or greater than 30 in the vaccinated cohort. However, individuals with BMI 25-29.9 had lower nAb levels as compared to normal and obese individuals for the infected group.
Taken together, the current study suggests that vaccination in pregnancy is highly effective in generating nAbs against all the SARS-CoV-2 variants included in the study. Although the vaccine-induced neutralizing activity is comparable to natural immunity, cord levels of nAb are higher after vaccination.
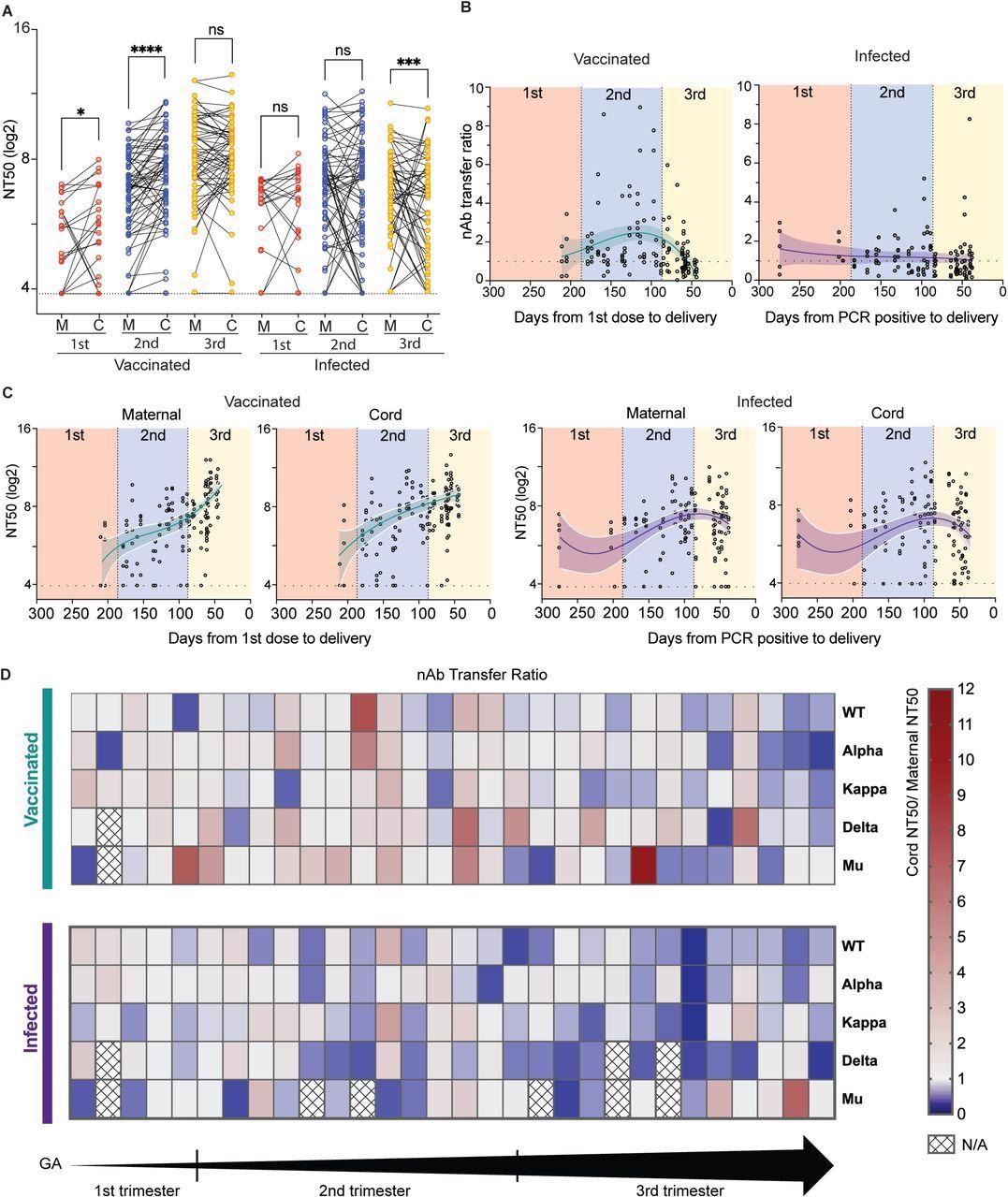 Placental Transfer of SARS-CoV-2 Antibodies. (A) The effect of trimester on antibody transfer. The dot plots show NT50 titers for a cohort of vaccinated maternal-cord pairs. Three separate analyses were performed on the trimester at the time of first exposure (Red, first trimester; blue, second trimester; yellow, third trimester). Lines connect mother: cord dyads. Significance was determined by the Wilcoxon signed-rank test. (B) Maternal-cord transfer ratios (TR) of nAbs were calculated by cord NT50/maternal NT50 to assess the efficiency of nAb transfer. nAb TRs are shown by timing on the first vaccine dose or the first positive PCR result. Correlations between the cord to maternal nAb ratio and days from events were analyzed using non-linear regression analysis. (C) Maternal and cord NT50 values were shown by timing on the first vaccine dose or the first positive PCR result. (D) Patient-based nAb TRs for each SARS-CoV-2 strain in both vaccinated and infected patients. All patients were ordered according to increasing gestational age (GA) within their groups. N/A, samples are not enough. *P < 0.05; ***P < 0.001; ****P < 0.0001; ns, not significant.
Placental Transfer of SARS-CoV-2 Antibodies. (A) The effect of trimester on antibody transfer. The dot plots show NT50 titers for a cohort of vaccinated maternal-cord pairs. Three separate analyses were performed on the trimester at the time of first exposure (Red, first trimester; blue, second trimester; yellow, third trimester). Lines connect mother: cord dyads. Significance was determined by the Wilcoxon signed-rank test. (B) Maternal-cord transfer ratios (TR) of nAbs were calculated by cord NT50/maternal NT50 to assess the efficiency of nAb transfer. nAb TRs are shown by timing on the first vaccine dose or the first positive PCR result. Correlations between the cord to maternal nAb ratio and days from events were analyzed using non-linear regression analysis. (C) Maternal and cord NT50 values were shown by timing on the first vaccine dose or the first positive PCR result. (D) Patient-based nAb TRs for each SARS-CoV-2 strain in both vaccinated and infected patients. All patients were ordered according to increasing gestational age (GA) within their groups. N/A, samples are not enough. *P < 0.05; ***P < 0.001; ****P < 0.0001; ns, not significant.
Vaccination 60 days before delivery is important for the efficient transfer of the antibodies to the neonates. The results of this study, therefore, strengthen current recommendations of vaccinating all pregnant women globally, as it would not only protect the mother but also the newborn against SARS-CoV-2 infection.
Limitations
The study had certain limitations. First, the number of patients exposed in the first trimester was much less than the other two trimesters. Second, the sequencing data for the natural infection cohort is not available. Third, detailed antibody characterization was not carried out.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Matsui, Y., Li, L., Prahl, M., et al. (2021). Neutralizing Antibody Activity Against SARS-CoV-2 Variants in Gestational Age-Matched Mother-Infant Dyads. medRxiv. doi:10.1101/2021.12.09.21267557. https://www.medrxiv.org/content/10.1101/2021.12.09.21267557v1.
- Peer reviewed and published scientific report.
Matsui, Yusuke, Lin Li, Mary Prahl, Arianna G. Cassidy, Nida Ozarslan, Yarden Golan, Veronica J. Gonzalez, et al. 2022. “Neutralizing Antibody Activity against SARS-CoV-2 Variants in Gestational Age–Matched Mother-Infant Dyads after Infection or Vaccination.” JCI Insight 7 (12). https://doi.org/10.1172/jci.insight.157354. https://insight.jci.org/articles/view/157354.