As of February 28, 2022, coronavirus disease 2019 (COVID-19) has caused over 5.95 million deaths worldwide, with especially high rates of mortality reported among the elderly, frail, and immunocompromised.
Kidney transplant recipients are among the most at-risk individuals of COVID-19 due to the need for long-term immunosuppressive medication to avoid rejection. Their response to vaccination is also poor, and researchers continue to examine methods to achieve a better vaccine response in this population.

Study: Three-Month Follow-Up of Heterologous Vs Homologous Third Vaccination in Kidney Transplant Recipients. Image Credit: Niphon Subsri / Shutterstock.com

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
A new study published on the preprint server medRxiv* assesses the comparative neutralizing antibody level elicited by a homologous three-dose two messenger ribonucleic acid (mRNA) vaccine regimen to a heterologous COVID-19 vaccine regimen that included two mRNA vaccine doses followed by a third viral vector vaccine booster dose in kidney transplant recipients.
Introduction
Previously, the authors of the current study had carried out a single-blinded trial of homologous and heterologous vaccination in kidney transplant recipients. About 40% of patients who had previously received two doses of an mRNA vaccine had anti-spike antibodies at four weeks from the third dose, irrespective of whether this was with an mRNA vaccine or an adenoviral vector vaccine.
This contradicted other studies that suggest a higher CD4+ T-cell anti-SARS-CoV-2 spike response and anti-spike antibody response with a heterologous booster compared to a homologous one in patients who had received a viral vector vaccine or B-cell depleting therapy.
The focus of the current study was to assess this finding, as well as examine how antibody and T-cell levels rose after the third dose beyond four weeks, and determine the direction of change after the first month.
Study findings
The current study included 85 patients each in the mRNA and vector vaccine booster groups. These patients were on immunosuppressive medications like belatacept, tacrolimus, corticosteroids, cyclosporin, and azathioprine. There were eight deaths, four in each group, all in patients who showed low or no response to the vaccine.
At one month, about 40% of patients in each group showed seroconversion for anti-SARS-CoV-2 antibodies. This rose to approximately 50% of the patients in either group at three months from the third dose.
Absolute antibody titers were similar between the mRNA and vector vaccine booster groups at 0.2 U/mL and 0.81 U/mL, respectively. The difference was observed at higher cut-offs at three months. At this point, using threshold levels above 141 and 264 BAU/mL, 4% and 1% of patients in the mRNA booster group achieved the cut-offs as compared to 15% and 10% in the vector vaccine group, respectively.
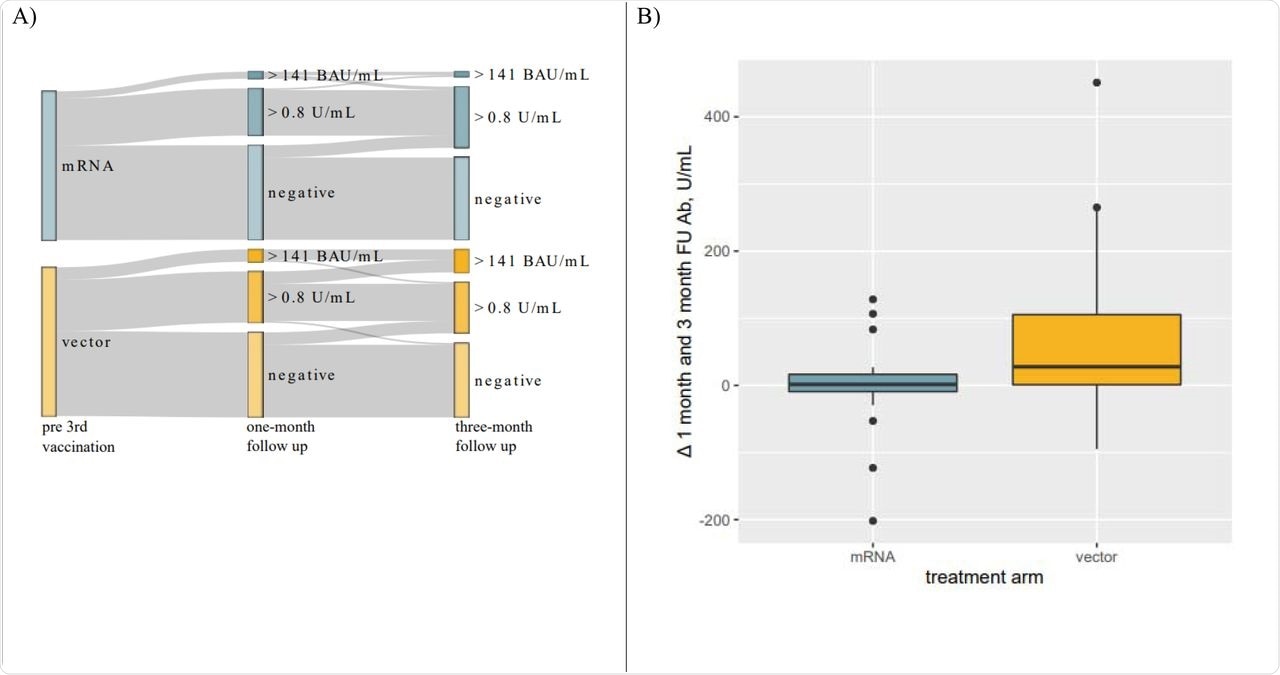
Panel A: Sankey Diagram visualizing changes in response rate to 3rdvaccination. A significantly larger proportion of individuals developed antibody levels > 141 BAU/mL. Panel B: Boxplots visualizing changes in antibody levels from one-to three-month FU in patients who seroconverted within one-month after receiving their 3rd vaccination. Antibody levels in individuals receiving a heterologous 3rd vaccination further increased while remaining unaltered in patients receiving mRNA vaccines.
The odds were thus in favor of the vector vaccine group, by 5- and about 9-fold, respectively. However, this difference was not obvious at one month.
Almost 10% of patients who were seronegative at one month seroconverted by three months, and all, except one in the vector vaccine group, had antibody levels greater than 0.8 U/mL at three months. Antibody levels in the mRNA group remained stable after one month; however, these levels continued to rise in the vector vaccine group until reaching a median of 25.8 U/mL and 77.7 U/mL at the three-month point, respectively.
T-cell responses at one month were comparable among the top responders in each group. CD4+ and CD8+ T-cell responses to SARS-CoV-2 were observed in 83% and 36% of patients, respectively.
Overall, 0.03% and 0.003% of total CD4+ and CD8+ T-cells, respectively, were specifically directed against SARS-CoV-2. No significant difference was observed between the groups.
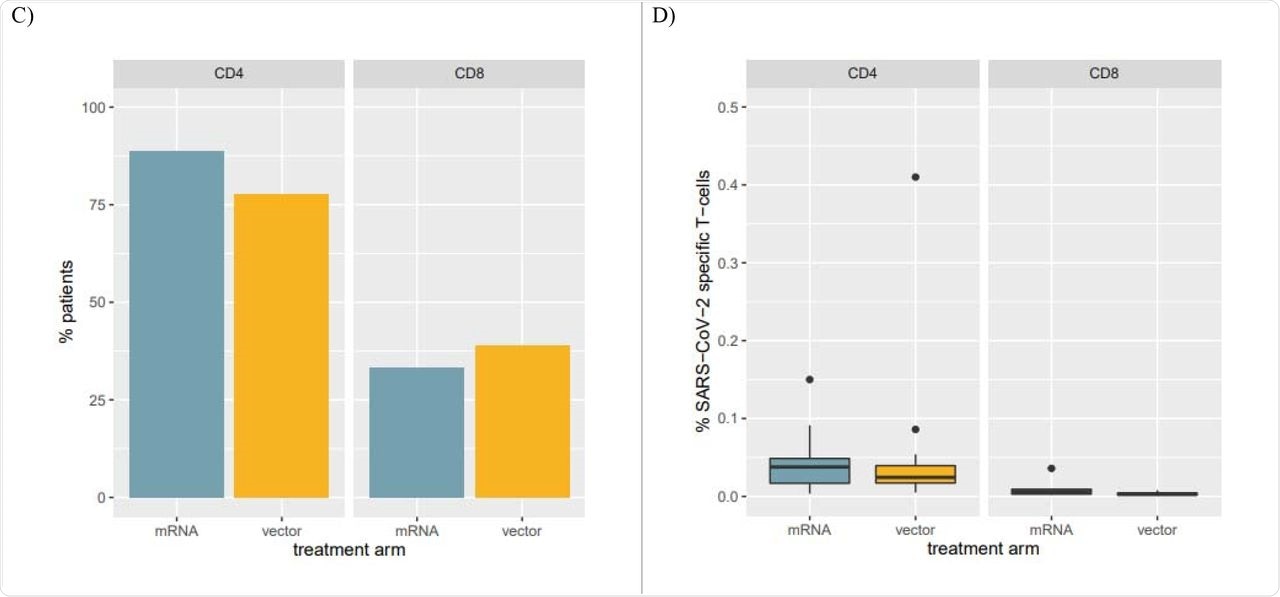
Panel C: Percentage of patients with SARS-CoV-2 specific CD4 and CD8 T-cells among the top humoral responders at the one-month FU. Panel D: Percentages of SARS-CoV-2 specific T-cells in patients with SARS-CoV-2 specific CD4 and CD8 T-cells.
Implications
The current study demonstrated that in kidney transplant recipients who had completed a primary series of mRNA vaccination, a homologous third dose booster elicited antibody responses that remained stable after one month and up to three months. Conversely, a heterologous third dose administration using a vector vaccine led to a rising antibody titer over this period, which resulted in overall higher antibody levels in this group.
Therefore, a greater proportion of patients in the heterologous booster group had antibody levels above the neutralizing threshold compared to the homologous booster group, even though the seroconversion rates were comparable in both groups.
Cellular response parameters remained comparable in both groups at four weeks, unlike earlier reports suggesting a higher level of T-cell response after heterologous boosters. However, no clear trend was observable with respect to antibody titers in these studies.
Despite similar overall seroconversion rates and comparable antibody levels at four weeks, heterologous 3rd boost vaccination using Ad26COVS1 results in significantly higher antibody levels but not CD4 or CD8 responses in [kidney transplant recipients] over a three-month follow-up period compared to additional homologous vaccination.”
The current study findings are important in the presence of new variants that show immune escape capability, as this may be overcome by higher antibody levels that protect against a broader range of variants.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.