Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causal agent of the COVID-19 pandemic, has evolved throughout the pandemic into novel variants of concern. The risk of the emergence of new variants in the future persists as the virus continues to evolve. The main target of the neutralizing antibodies (nAbs) is the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein. As the virus binds with the host receptor through its S protein for cell entry, SARS-CoV-2 vaccines are designed with S protein as the immunogen. Anti-S antibodies increase after vaccination but drop significantly over time. Hence, booster vaccine doses were approved to ensure the circulation of antibodies.
The National Institutes of Health (NIH) initiated a study termed SPARTA (SARS seroprevalence and respiratory tract assessment) to understand whether infection- or vaccination-induced immunity protects against future infections.
The study
The current study examined the rate of the waning of antibodies in a large cohort that comprised infected and vaccinated individuals. Participants were immunized with mRNA vaccines from Pfizer (BNT162b2 vaccine) and Moderna (mRNA-1273 vaccine). In addition, the researchers included about 1081 SPARTA participants through random selection. Enzyme-linked immunosorbent assays (ELISA) were performed using heat-inactivated serum samples and detected IgG antibodies using horseradish peroxidase (HRP) conjugated with goat anti-human IgG detection antibody. Virus neutralization (VN) tests were carried out by co-incubating SARS-CoV-2 (USA-WA1/2020 strain) with serum samples for an hour and later added to Vero E6 cells monolayer and observing for cytopathic effects (CPEs).
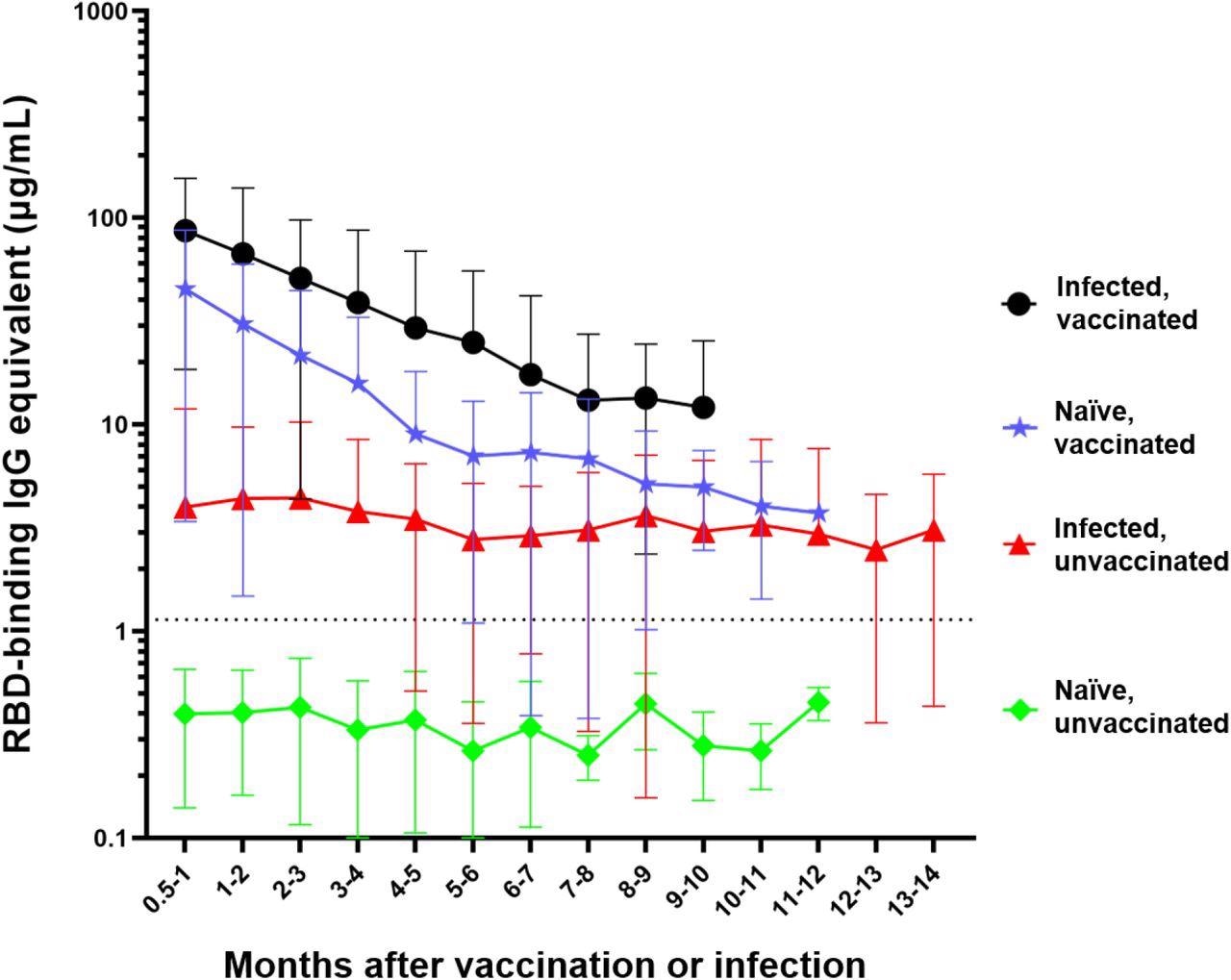
Differential waning of RBD-binding IgG antibody levels based on vaccination and infection status. Naïve unvaccinated (n=418) and infected unvaccinated (n=306) show no change in antibody levels over time (p>0.05); naïve, vaccinated participants (n=515) and infected, vaccinated participants (n=303) both show significant waning over the time (****p<0.0001). The antibody level of the naïve unvaccinated group was always lower than the other groups (****p<0.0001); the infected vaccinated group was always higher than any other group (*p<0.05); naïve, vaccinated group is higher than infected, unvaccinated group for the first 4 months after vaccination (**p<0.0014). The rate of decay was only significantly different between the vaccinated and infected groups (****p<0.0001) but not between the two vaccinated (p=0.7762) and the two unvaccinated groups (p=0.9476). Number of months start with the time of reception of the primary vaccines series for the vaccinated groups, time of infection for the infected unvaccinated group, and the first available timepoint for the naïve unvaccinated group.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Memory B lymphocytes were differentiated from peripheral blood mononuclear cells (PBMCs). The cell culture supernatants were assessed for total (non-antigen specific) and antigen-specific IgG levels. Total IgG levels were quantified to ascertain successful in vitro cell differentiation resulting in de novo memory B cell recall. RBD- and S-specific IgG antibody levels and S-specific IgA and IgM levels were quantified. A one-way analysis of variance (ANOVA) and paired t-tests were conducted to compare four groups, i.e., non-vaccinated naïve, non-vaccinated infected, vaccinated naïve, and vaccinated infected.
Results
The study comprised 418 subjects who were non-vaccinated and infection-naïve, 306 non-vaccinated infected individuals, 515 vaccinated naïve participants, and 303 vaccinated people with documented COVID-19 history. Mean antibody levels in the naïve non-vaccinated subjects were 0.4 µg/ml, lower than the threshold, and remained consistent without significant changes over time. Infected individuals who were not vaccinated demonstrated IgG antibodies at 4 µg/ml concentration initially without any significant antibody waning through the 14 months post-infection. The antibody responses were initially more significant in SARS-CoV-2-naïve vaccinated subjects at a 44.8 µg/ml concentration, which declined significantly after six months of vaccination. The seroconversion was even higher in those vaccinated post-infection with a mean concentration of 86 µg/ml that dropped significantly after five months.
Expectedly, anti-RBD IgG levels were the lowest in infection-naïve, non-vaccinated subjects. People vaccinated after infection had significantly higher anti-RBD antibodies than others. Only 306 participants were boosted with a third dose, either with a homologous or heterologous vaccine, with a majority receiving homologous doses. The researchers observed that antibody levels were increased by 14 times after receiving the booster dose. While antibody waning post-booster vaccination was evident, the antibody levels were significantly higher after five months than before receiving the booster.
Across the entire cohort, 25 individuals were positive for RBD antibodies during the study, but the antibody levels dropped below the threshold sometime later. VN tests were performed to confirm their lost seroprotective status. Serum from 13 subjects demonstrated in vitro differentiation upon stimulation with recombinant IL-2 and R848, recalling the cellular memory responses. Of the samples from 10 participants who lost seroprotective status post-vaccination, seven demonstrated significant anti-RBD and anti-S memory recall while the other three did not. None of the three participants who lost seroprotection post-infection showed any (significant) memory recall.
Conclusions
The study observed distinct differences in antibody waning across the participants. Although the infection-elicited antibody levels were lower than vaccine-induced levels, antibody waning was not significant for 14 months post-infection, while vaccine-induced antibody levels dropped quickly despite high initial levels. Infected and vaccinated participants had significantly higher antibody levels for over 10 months than non-vaccinated infected individuals.
In the six participants with robust memory recall, B cell immunophenotyping confirmed the presence of IgG+ memory B lymphocytes in significant numbers. To conclude, vaccinated people seroconverted to higher antibody levels with significant antibody waning. A booster dose increased the peak antibody responses indicating that high circulating antibody levels could effectively counteract and prevent antibody waning.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
The effect of waning on antibody levels and memory B cell recall following SARS-CoV-2 infection or vaccination, David Forgacs, Vanessa S. Moraes, Hannah B. Hanley, Jasper L. Gattiker, Alexandria M. Jefferson, Ted M. Ross bioRxiv 2022.03.16.484099; doi: https://doi.org/10.1101/2022.03.16.484099, https://www.biorxiv.org/content/10.1101/2022.03.16.484099v1
- Peer reviewed and published scientific report.
Forgacs, David, Vanessa Silva-Moraes, Giuseppe A. Sautto, Hannah B. Hanley, Jasper L. Gattiker, Alexandria M. Jefferson, Ravindra Kolhe, and Ted M. Ross. 2022. “The Effect of Waning on Antibody Levels and Memory B Cell Recall Following SARS-CoV-2 Infection or Vaccination.” Vaccines 10 (5): 696. https://doi.org/10.3390/vaccines10050696. https://www.mdpi.com/2076-393X/10/5/696.