Two years since the coronavirus disease 2019 (COVID-19) pandemic, SARS-CoV-2 surveillance has been challenging in the United States (US). Polymerase chain reaction (PCR) assays were deployed slowly, and the massive demand for PCR-based diagnosis constrained laboratories and supply chains. While point-of-care antigen tests were available by late 2020, their application was not widespread until the emergence of the SARS-CoV-2 Omicron variant.
The US recorded more than 1.3 million cases on January 10, 2022, the highest ever single-day surge, with a seven-day average being three times higher than the previous peak. As a result, laboratory testing was overwhelmed by the massive, unprecedented increase of COVID-19 cases. Therefore, there is a pressing need to diversify and explore novel viral testing strategies to assess the transmission risk of SARS-CoV-2.
 Study: SARS-CoV-2 and other respiratory pathogens are detected in continuous air samples from congregate settings. Image Credit: Kateryna Kon / Shutterstock
Study: SARS-CoV-2 and other respiratory pathogens are detected in continuous air samples from congregate settings. Image Credit: Kateryna Kon / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study and findings
In the present study, researchers tested if active air samplers could be employed for prospective air surveillance of SARS-CoV-2.
The research team deployed continuous air samplers in many public places between July 19, 2021, and February 9, 2022, for surveying SARS-CoV-2 at sites that are flagged as high risk for indoor transmission and close-contact.
Thermo Fisher Scientific's aerosol sense samplers were used for weekly and daily surveillance. Transcription-mediated amplification (TMA) and quantitative reverse transcription PCR (RT-qPCR) were performed on the tested samples for the extraction of SARS-CoV-2 ribonucleic acid (RNA). The testing locations throughout Wisconsin and Minnesota covered a hospital, office, two preschools, cafeteria, brewery taproom, campus coffee shop, athletic training facility, bar, two shelters, and four K-12 schools.
About 527 air samples were collected from 15 locations, with a majority (88.4%) from Dane County, Wisconsin. The authors detected 106 SARS-CoV-2-positive air samples, and 52 others were deemed inconclusive.
Despite the intensive implementation of risk mitigation measures, air cartridges were positive for SARS-CoV-2 at 14 surveillance locations. Since, through this strategy, it was impossible to determine the COVID-19 status of every individual vicinal to air samplers, a retrospective correlation with air surveillance data was estimated with reported COVID-19 infection cases at one of the sites.
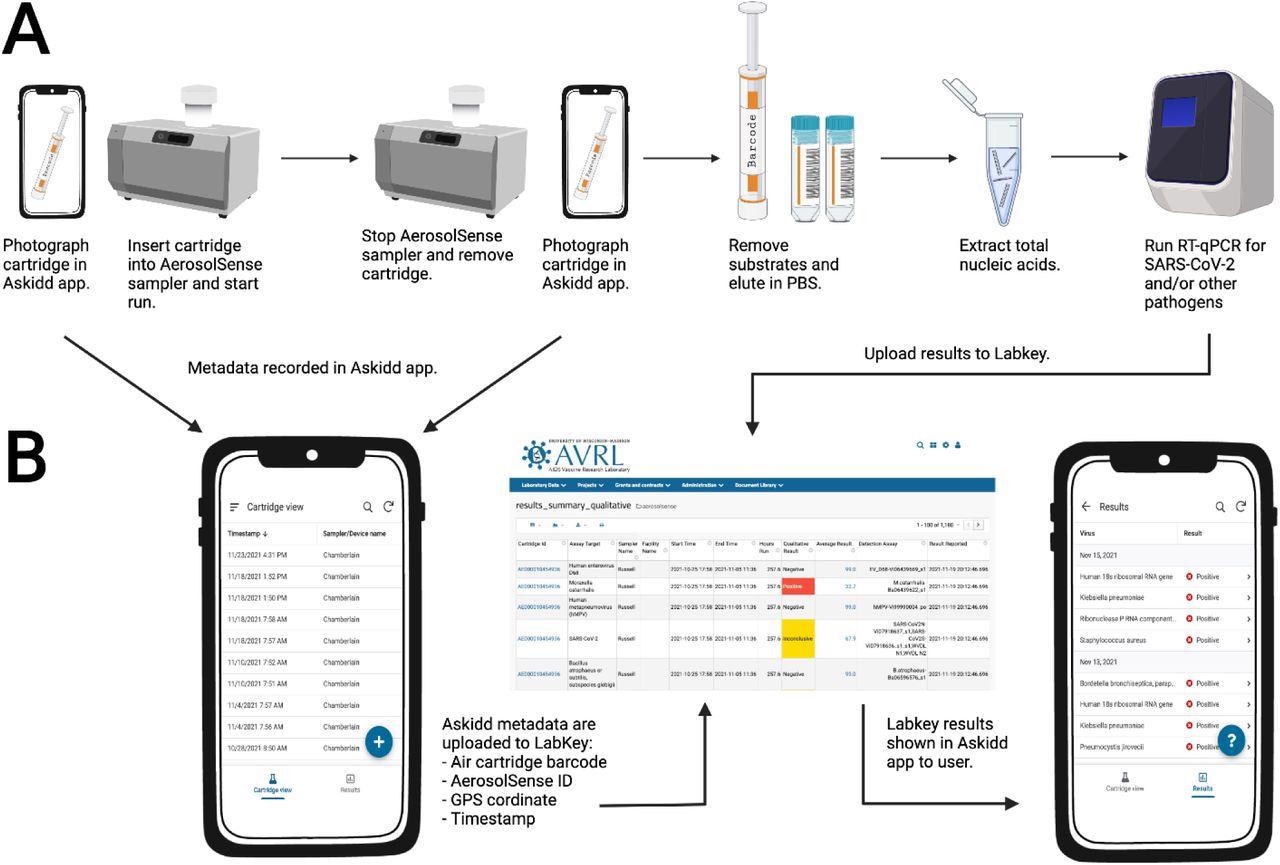
Air sample testing workflow.
(A) Overview of air sample collection, processing, and testing. (B) Air sample data collection and management. Individuals in charge of changing air cartridges at surveillance sites use the iOS and Android Askidd mobile app to collect metadata on air samples when cartridges are inserted and removed. Data are compiled in Labkey database and displayed to surveillance sites in the Askidd mobile app. Created with BioRender.com.
The first documented case was from a quarantining individual who took the test after developing symptoms. Those present in the same room had to quarantine, but none were positive at the time. Three close contacts tested positive with antigen tests. Subsequently, several others tested positive, resulting in an outbreak of 20 confirmed infections. Air samples collected at least seven days before the first documented case of COVID-19 were positive.
Next, the researchers tested the feasibility of extending the interval of sampling. For five weeks, two samplers were deployed to capture specimens weekly or daily. The team noted that either weekly or daily sampling could be used in congregate settings with a balance between turnaround time and cost. Additionally, the air samples collected between October 25, 2021, and February 9, 2022, from eight locations in Wisconsin were tested for the presence of other respiratory pathogens (bacteria, fungi, and viruses). During this period, 16 other respiratory pathogens were detected. Commensals or transiently commensal respiratory microbes, including Klebsiella pneumoniae, Moraxella catarrhalis, and Staphylococcus aureus, were identified frequently. Pathogens such as adenovirus, cytomegalovirus, Epstein-Barr virus, influenza, and parainfluenza, among others, causing illness in school-aged children were also detected. The nucleic acids of influenza A virus (IAV) were detected in air specimens on the University of Wisconsin-Madison campus starting from November 10, 2021, through January 2022. One of the two samplers placed in the campus coffee shop provided negative air samples beginning the week of December 22, 2021, until January 12, 2022, coinciding with the fall break.
Lastly, SARS-CoV-2 genomic sequencing was performed on 11 air samples with low cycle threshold (Ct) values collected from sites where people were often unmasked to eat or drink. Using two sequencing strategies, partial or near-full length genome sequences were obtained. Samples collected between December 30, 2021, and January 25, 2021, contained the characteristic mutations of the SARS-CoV-2 Omicron BA.1 lineage, coincident with the advent of the Omicron variant in the region.
Conclusions
The study findings demonstrated the applicability of active air samplers for surveillance of SARS-CoV-2 and other respiratory pathogens in addition to existing mitigation strategies. Air surveillance might help identify pathogenic aerosols in congregate settings to assess the transmission risk. It is noteworthy that RT-qPCR testing does not distinguish between an infectious virus or the genetic material. The researchers did not isolate SARS-CoV-2 or other pathogens from positive samples through cultures.
Air surveillance could be a scalable, cost-effective strategy and prove a high-throughput substitute for individual testing to detect SARS-CoV-2 and other respiratory pathogens in congregate settings. Thus, establishing an air surveillance network similar to the wastewater surveillance system might offer additional safeguards and improve resilience to future threats of respiratory disorders.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
SARS-CoV-2 and other respiratory pathogens are detected in continuous air samples from congregate settings, Mitchell D Ramuta, Christina M Newman, Savannah F Brakefield, Miranda R Stauss, Roger W Wiseman, Amanda Kita-Yarbro, Eli J O'Connor, Neeti Dahal, Ailam Lim, Keith P Poulsen, Nasia Safdar, John A Marx, Molly A Accola, William M Rehrauer, Julia A Zimmer, Manjeet Khubbar, Lucas J Beversdorf, Emma C Boehm, David Castaneda, Clayton Rushford, Devon Arch Gregory, Joseph D Yao, Sanjib Bhattacharyya, Marc C Johnson, Matthew T Aliota, Thomas Friedrich, David O'Connor, Shelby L O'Connor, medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.03.29.22272716, https://www.medrxiv.org/content/10.1101/2022.03.29.22272716v1
- Peer reviewed and published scientific report.
Ramuta, Mitchell D., Christina M. Newman, Savannah F. Brakefield, Miranda R. Stauss, Roger W. Wiseman, Amanda Kita-Yarbro, Eli J. O’Connor, et al. 2022. “SARS-CoV-2 and Other Respiratory Pathogens Are Detected in Continuous Air Samples from Congregate Settings.” Nature Communications 13 (1): 4717. https://doi.org/10.1038/s41467-022-32406-w. https://www.nature.com/articles/s41467-022-32406-w.