The coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 has put an unprecedented burden on the global healthcare system, with more than 510 million infections and over 6.22 million deaths worldwide. Although the fast-paced development of effective vaccines has significantly controlled the pandemic trajectory, the unavailability of specific antiviral therapeutics has become a significant public health concern.
Besides repurposing clinically-approved antiviral medicines and developing novel small molecular inhibitors, several studies have investigated the antiviral efficacy of plant-derived bioactive compounds against COVID-19. These studies have indicated that a direct interaction between plant products and viruses can inhibit viral entry or early-stage disruption of viral replication.
In the current study, the scientists have investigated the antiviral efficacy of the extract of an herbal plant, Hypericum perforatum against SARS-CoV-2 infection. The antiviral efficacy of this plant has already been observed against the herpes simplex virus, human cytomegalovirus, hepatitis B virus, influenza A virus, and human immunodeficiency virus.
Specifically, the scientists have studied whether a quantified dry extract of Hypericum perforatum and its two active constituents naphtodianthrone hypericin and pseudohypericin can block infections caused by SARS-CoV-2 and its variants.
Important observations
The analysis of viral-blocking efficacy using a pseudo-typed vesicular stomatitis virus (VSV)-harboring SARS-CoV-2 spike protein revealed that the plant extract is capable of dose-dependently inhibiting SARS-CoV-2 without causing any cytotoxic effects.
Five main compounds of the plant were selected and investigated to identify the specific inhibitors of the virus. These compounds included naphtodianthrones hypericin and pseudohypericin, phloroglucinol derivative hyperforin, proanthocyanidin/condensed tannins procyanidin C1, and flavonol glycoside (quercetin-3-O-glucuronid). The analysis revealed that the treatment with hypericin and pseudohypericin leads to complete and 82% inhibition of the virus, respectively. However, no impact of other tested compounds on viral entry was observed. Moreover, no significant cytotoxic effects of hypericin and pseudohypericin were observed.
When tested against genuine infectious SARS-CoV-2, both the plant extract and its active compounds hypericin and pseudohypericin showed similar antiviral activity as observed against the pseudo-typed virus. In addition, both the plant extract and hypericin showed potent antiviral activity against the alpha, beta, delta, and omicron variants of SARS-CoV-2.
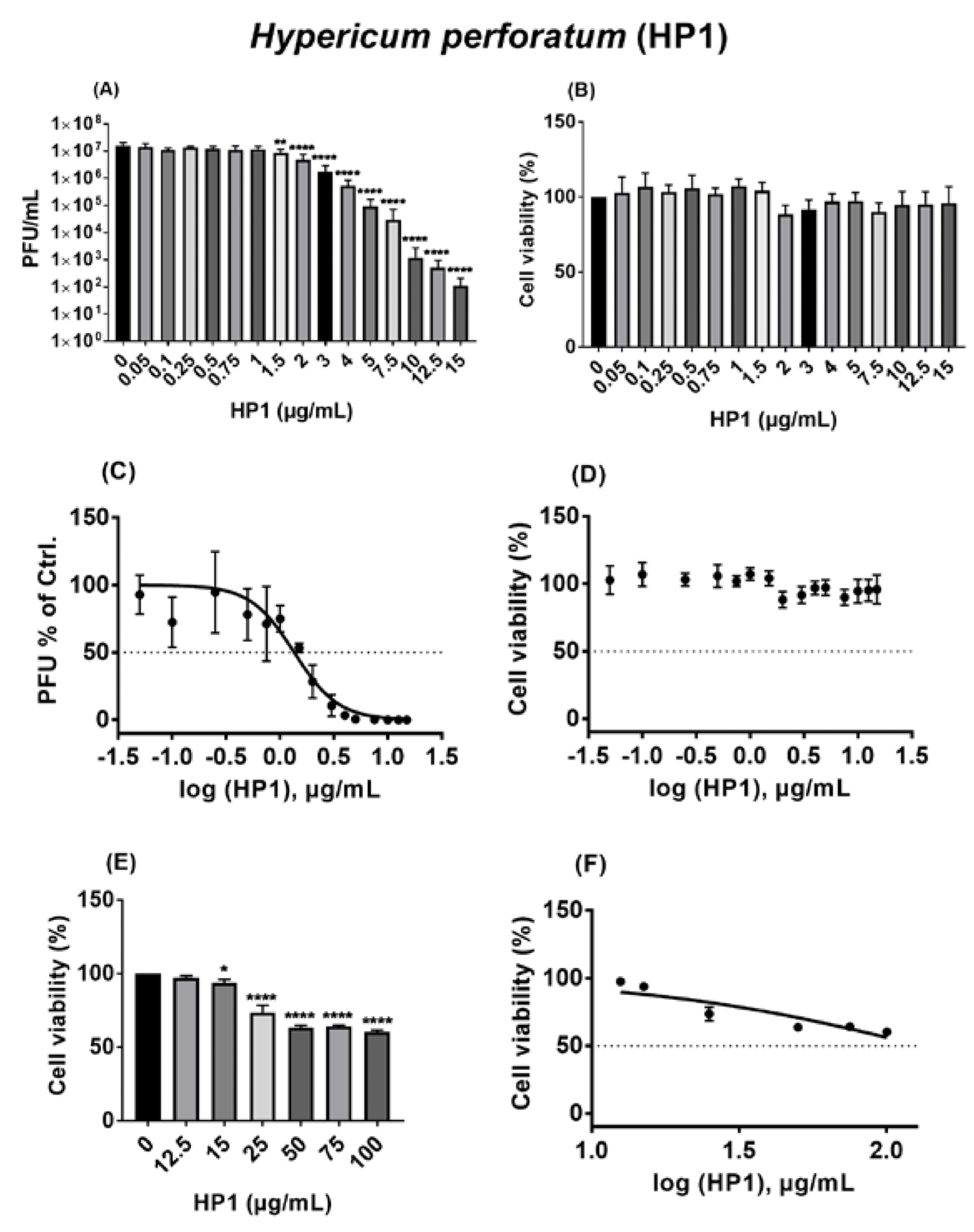 Hypericum perforatum (HP1) acts as an antiviral against ancestral SARS-CoV-2. (A,C) Vero cells were seeded overnight, and on the next day, prior to infection (MOI = 0.05), cells were incubated at 37 °C for 1h with infection-DMEM containing either solvent control (DMSO) or HP1. Concurrently, SARS-CoV-2 was incubated for 1 h at room temperature in infection-PBS that contained either DMSO or HP1. After infection (37 °C/1 h), cells were further incubated in infection-DMEM including either DMSO or HP1. After 24 h, virus supernatants were collected and subjected to plaque assay. (A) Results are expressed as PFU/mL (mean and s.d.), and one-way ANOVA with Dunnett’s multiple comparisons was done by comparing each value with the control. (C) Dose–response curve of the normalized virus titer values as % of solvent control is depicted (mean and s.d.). (B,D–F) Vero cells were seeded overnight, and on the next day, cells were incubated for 24 h with infection-DMEM that contained either solvent control (DMSO) or HP1. After incubation, the MTT assay-based cytotoxicity was measured. (B,E) Cell viability as % of solvent control is shown (mean and s.d.), and one-way ANOVA with Dunnett’s multiple comparisons was done by comparing each value with the control. (D,F) Dose–response curve of the normalized cytotoxicity values as % of solvent control is depicted (mean and s.d.). * for p ≤ 0.05, ** for p ≤ 0.01, and **** for p ≤ 0.0001
Hypericum perforatum (HP1) acts as an antiviral against ancestral SARS-CoV-2. (A,C) Vero cells were seeded overnight, and on the next day, prior to infection (MOI = 0.05), cells were incubated at 37 °C for 1h with infection-DMEM containing either solvent control (DMSO) or HP1. Concurrently, SARS-CoV-2 was incubated for 1 h at room temperature in infection-PBS that contained either DMSO or HP1. After infection (37 °C/1 h), cells were further incubated in infection-DMEM including either DMSO or HP1. After 24 h, virus supernatants were collected and subjected to plaque assay. (A) Results are expressed as PFU/mL (mean and s.d.), and one-way ANOVA with Dunnett’s multiple comparisons was done by comparing each value with the control. (C) Dose–response curve of the normalized virus titer values as % of solvent control is depicted (mean and s.d.). (B,D–F) Vero cells were seeded overnight, and on the next day, cells were incubated for 24 h with infection-DMEM that contained either solvent control (DMSO) or HP1. After incubation, the MTT assay-based cytotoxicity was measured. (B,E) Cell viability as % of solvent control is shown (mean and s.d.), and one-way ANOVA with Dunnett’s multiple comparisons was done by comparing each value with the control. (D,F) Dose–response curve of the normalized cytotoxicity values as % of solvent control is depicted (mean and s.d.). * for p ≤ 0.05, ** for p ≤ 0.01, and **** for p ≤ 0.0001
Mode of antiviral action
A series of experiments was conducted to determine the antiviral mode of action of the plant extract and its most active compound hypericin. The findings revealed that the pre-treatment of SARS-CoV-2 with the plant extract or hypericin before infection significantly reduces viral titers. Furthermore, the treatment of cells with the same compounds after SARS-CoV-2 infection also caused a decrease in viral titers; however, the degree of inhibition was comparatively lower in the later condition.
With further analysis, it was observed that the pre-treatment of the virus with plant extract or hypericin leads to a significant reduction in the expression of SARS-CoV-2 nucleocapsid, indicating the direct effect of both compounds on viral particle infectivity. However, no effects of these compounds were observed on the expression or function of SARS-CoV-2 spike protein.
Study significance
The study identifies Hypericum perforatum plant extract and its active compound hypericin as potent inhibitors of SARS-CoV-2 and its variants alpha, beta, delta, and omicron. Both compounds directly block viral infectivity at an early stage of infection, leading to a significant reduction in viral titers. However, no impact of the compounds on SARS-CoV-2 spike-mediated viral entry has been observed in the study.
Although pre-treatment of SARS-CoV-2 with the compounds before the infection has resulted in the highest antiviral activity, both compounds have also shown potent virus-blocking activity in already-infected cells. This highlights the possibility of an additional intracellular mode of action of the compounds.
Given the acceptable safety profile and potent antiviral efficacy, Hypericum perforatum and its active compounds could be considered as potential therapeutic as well as prophylactic agents against COVID-19.