Recently, the United States Food and Drug Administration (FDA) approved the use of a third booster dose with either the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 coronavirus disease 2019 (COVID-19) vaccines in immunocompromised individuals. This decision was made in an effort to prevent the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as mitigate waning immunity in vaccinated individuals.
Background
The emergence of the immune-evasive SARS-CoV-2 Omicron (B.1.1.529) variant is associated with reduced neutralizing antibody responses. This has prompted researchers to explore potential methods to improve the protection offered by current COVID-19 vaccines through booster vaccination.
A new Vaccines journal study compares the cross-protective humoral responses to different SARS-CoV-2 variants that can be induced either by vaccination or infection with the SARS-CoV-2 variant. Herein, researchers analyzed the neutralizing antibody response against the SARS-CoV-2 wild-type (WT), Delta, and Omicron strains among vaccinated individuals who were infected with the Omicron variant or uninfected vaccine recipients, as well as among non-vaccinated individuals who were infected with the Omicron variant.
About the study
The current study included a total of 95 participants, 75 of whom were healthcare workers who had received the third dose of the mRNA-1273 (Moderna) vaccine following two doses of the BNT162b2 vaccine three months prior. Out of the 75 healthcare workers, 25 had a previous COVID-19 diagnosis confirmed both by reverse transcription-polymerase chain reaction (RT-PCR) assay and serological testing.
The remaining 20 participants were unvaccinated and infected by the SARS-CoV-2 Omicron BA.1 strain. Blood samples were collected from all participants for detection of SARS-CoV-2 spike receptor-binding domain (RBD) specific immunoglobulin G (IgG). Additionally, the sera of all the patients were tested to detect the presence of specific neutralizing antibodies against SARS-CoV-2 variants.
Furthermore, neutralizing antibody titers of unvaccinated individuals infected with the Omicron BA.1 strain were compared with individuals who were infected with the SARS-CoV-2 WT strain.
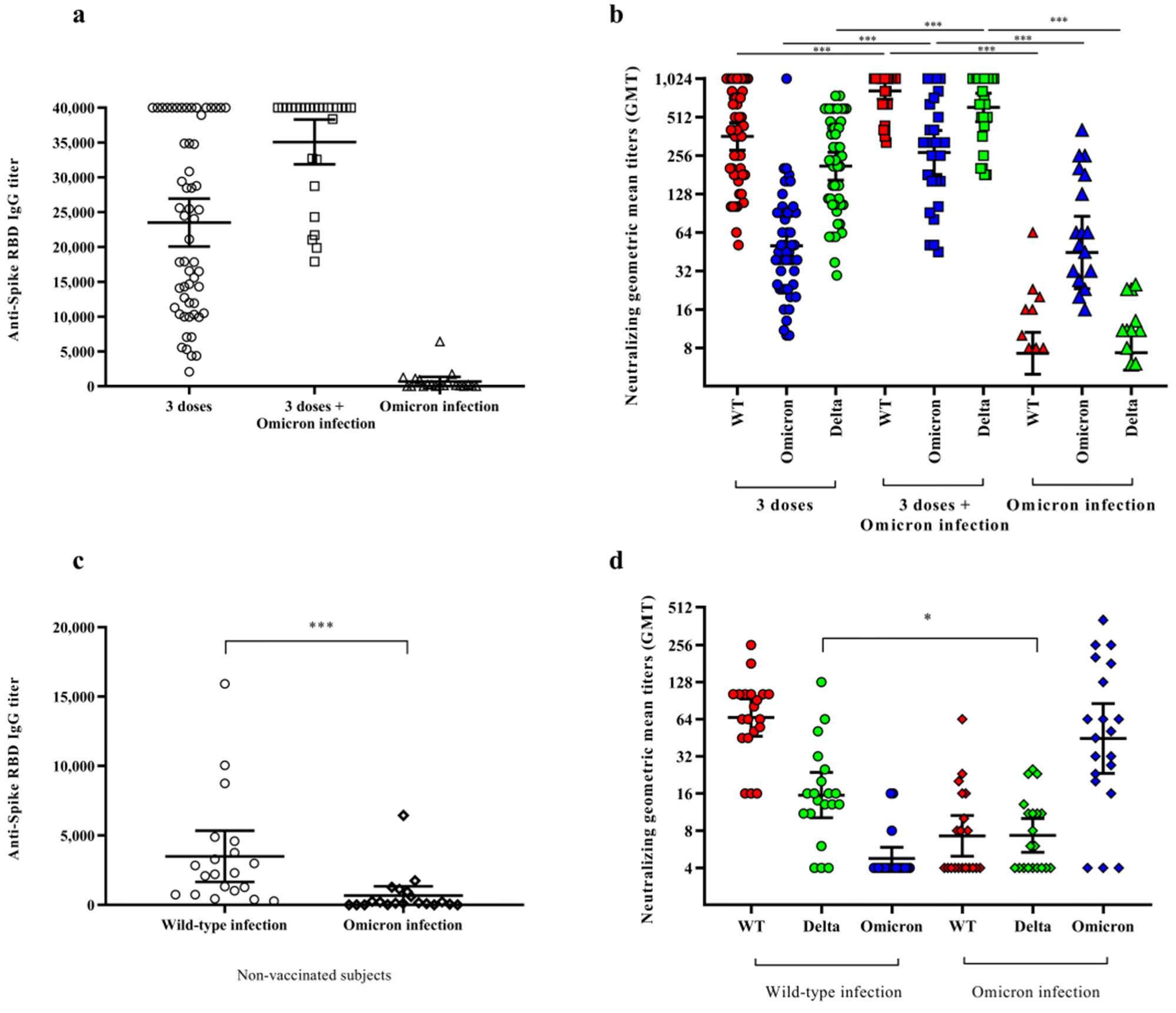 Immune response in vaccinated subjects, who either had or did not have a SARS-CoV-2 infection, and naturally infected subjects. Titers of anti-spike IgG antibodies (Panels (a,c)) and neutralizing SARS-CoV-2 antibodies (Panels (b,d)) in serum samples of subjects naturally infected with SARS-CoV-2 (triangles), vaccinated subjects with three doses of mRNA vaccine (circles), and vaccinees infected with the Omicron variant (squares). Differences in neutralizing IgG antibodies were evaluated against WT (red), Delta (B.1.617.2) (green), and Omicron (BA.1) (blue) strains (Panel (b)). Comparison of serum samples of WT infected subjects (circles) with those of Omicron infected subjects (rhombuses) against the three variants (Panel (d)). In each plot, the horizontal line represents the mean (Panels (a,c)) or the geometric mean (Panels (b,d)), while the top and bottom lines show the 95% confidence interval (CI 95%). The p values are reported in the figures, where * stands for p < 0.05 and *** stands for p < 0.001.
Immune response in vaccinated subjects, who either had or did not have a SARS-CoV-2 infection, and naturally infected subjects. Titers of anti-spike IgG antibodies (Panels (a,c)) and neutralizing SARS-CoV-2 antibodies (Panels (b,d)) in serum samples of subjects naturally infected with SARS-CoV-2 (triangles), vaccinated subjects with three doses of mRNA vaccine (circles), and vaccinees infected with the Omicron variant (squares). Differences in neutralizing IgG antibodies were evaluated against WT (red), Delta (B.1.617.2) (green), and Omicron (BA.1) (blue) strains (Panel (b)). Comparison of serum samples of WT infected subjects (circles) with those of Omicron infected subjects (rhombuses) against the three variants (Panel (d)). In each plot, the horizontal line represents the mean (Panels (a,c)) or the geometric mean (Panels (b,d)), while the top and bottom lines show the 95% confidence interval (CI 95%). The p values are reported in the figures, where * stands for p < 0.05 and *** stands for p < 0.001.
Study findings
Higher protective titers of both circulating IgG, as well as neutralizing antibody titers, were observed among participants who received the third booster dose. Omicron infection was found to induce a low level of anti-spike RBD IgG in naive individuals, while it significantly increased specific antibody levels in vaccinated individuals.
Anti-RBD IgG levels were much lower in individuals infected with Omicron as compared to those infected with the WT strain. Omicron infection was also found to generate neutralizing antibody responses against WT, Delta, and Omicron strains among vaccinated individuals, while this type of cross-protective role response was not observed in unvaccinated individuals. Furthermore, the antibodies of WT-infected individuals were found to cross-react with Delta but not Omicron, thus suggesting that Omicron antibodies cannot protect against WT or Delta strains.
Conclusions
Infection with the SARS-CoV-2 Omicron variant alone cannot protect against WT or Delta infection unless the individual is vaccinated. Thus, the findings from the current study suggest that the development of a new vaccine against the B1.1.529 and BA lineages would likely protect against the Omicron subvariants, as these strains are quite different from other SARS-CoV-2 variants.
Journal reference:
- Anichini, G., Terrosi, C., Gandolfo, C., et al. (2022). Omicron Infection Evokes Cross-Protection against SARS-CoV-2 Variants in Vaccinees. Vaccines. doi:10.3390/vaccines10050808.