While the RBD is typically considered immunodominant, several reports depicted that neutralizing antibodies also target the NTD. Engineering RBD and the S2 subunit was the main focus of earlier anti-COVID-19 therapeutic efforts. On the other hand, NTD has long been disregarded because of the incomplete comprehension of its biophysical limitations.
About the study
In the present study, the researchers quantified the impacts of thousands of single mutations of NTD on the S protein expression in SARS-CoV-2 by deep mutational scanning.
The scientists developed a mutant library that includes all potential single amino acid mutations spanning residues 14–301 of the S protein to explore how NTD mutations affect SARS-CoV-2 S protein expression. They examined the expression score distributions of silent, nonsense, and missense mutations to gauge the quality of the deep mutational scanning findings. The authors created a heatmap to encapsulate the expression ratings for each mutation.
The team's goal was to determine the biophysical factors that affect the mutational toleration of NTD residues considering the S protein expression. First, they inquired whether the relative solvent accessibility (RSA) and mutational tolerance were linked. The investigators next examined the NTD sequences of sarbecovirus strains, such as SARS-CoV-2, to evaluate whether the mutational tolerance was associated with sequence conservation.
The scientists estimated the distance between each NTD residue and the S protein's S2/RBD. They created G232E, S50Q, and S50Q/G232E double mutant human embryonic kidney 293 (HEK293T) cell lines using the same landing pad strategy. The authors examined the local contexts of G232E and S50Q on the S protein's structure and conducted structural modeling with Rosetta App to investigate the effects these mutations had on the protein's structural integrity.
The team further investigated if G232E, S50Q, and S50Q/G232E displayed a variation in fusion activity relative to the SARS-CoV-2 wild type (WT) strain to comprehend the functional implications of these mutations. They then explored if the S protein's antigenicity was affected by G232E, S50Q, and S50Q/G232E.
Results
The study results revealed that contrary to RBD residues, the NTD residues displayed a weak association between mutational tolerance and RSA. Instead, there was a significant correlation between NTD residues' ability to tolerate mutations and their distance from S2 and RBD. Hence, the authors found that the mutational tolerance of NTD residues was inversely linked with their distance from the S2 and RBD, considering the SARS-CoV-2 S protein expression.
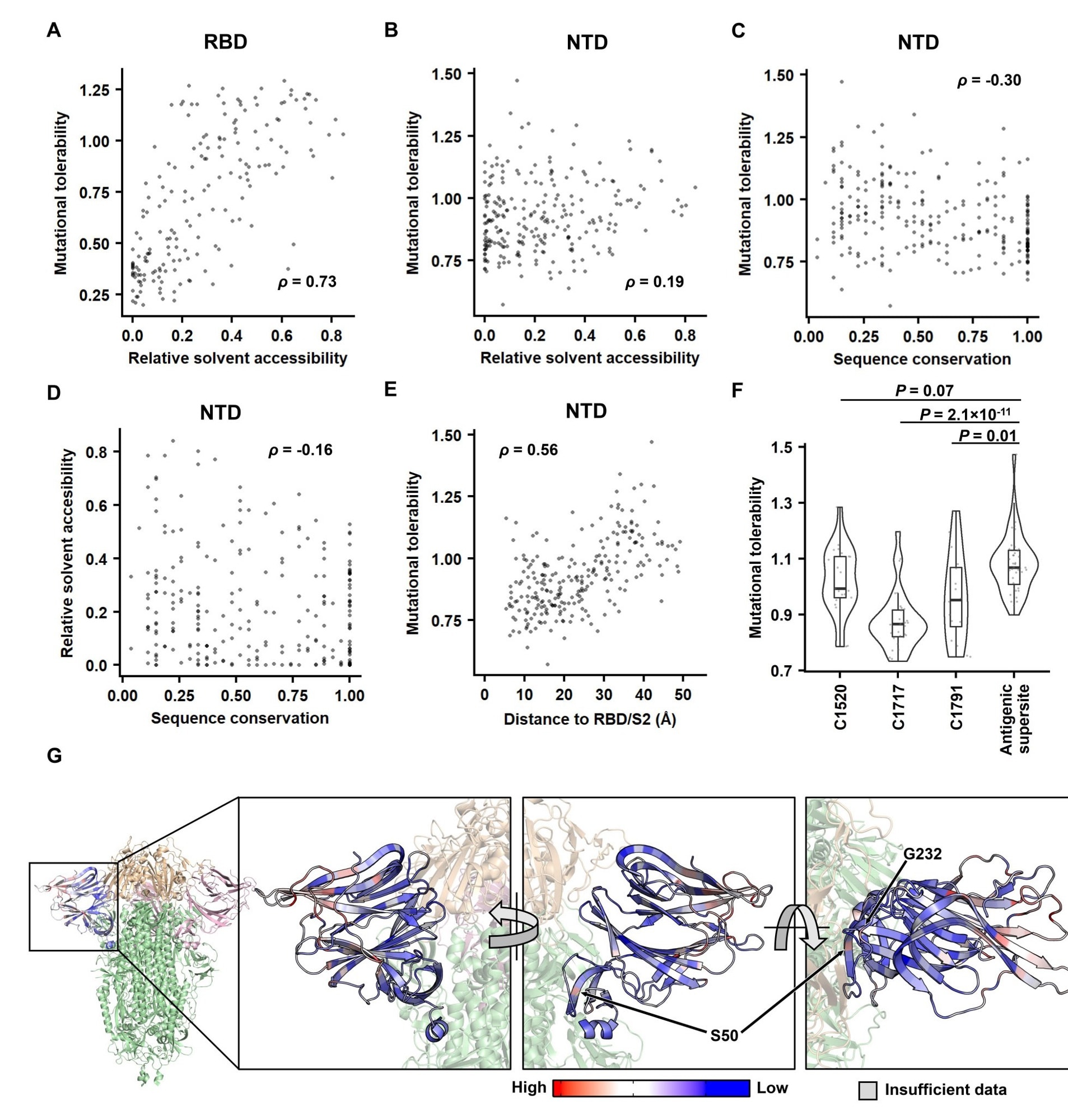 Identifying the biophysical determinants of mutational tolerability. (A-B) The relationship between relative solvent accessibility (RSA) and the mutational tolerability is shown for (A) RBD and (B) NTD. The deep mutational scanning data on RBD expression was from a previous study42. (C-D) The relationship between sequence conservation among 27 sarbecovirus strains and (C) the mutational tolerability, or (D) RSA of each NTD residue is shown. of each NTD residue is shown. (A-E) Each data point represents one residue. The Spearman’s rank correlation coefficient (ρ) is indicated. (F) The mutational tolerability of residues within the cross-neutralizing NTD antibody epitopes (C1520, C1717, C1791)16 is compared to that within the antigenic supersite14 using a violin plot. Each data point represents one residue. P-values were computed by a two-tailed t-test. (G) The mutational tolerability of each NTD residue is projected on one NTD of the S trimer structure (PDB 6ZGE44 and PDB 7B6245). Red indicates residues with higher mutational tolerability, while blue indicates residues with lower mutational tolerability. Residues with insufficient data to calculate mutational tolerability are colored in grey. Two residues of interest, namely S50 and G232, are indicated. RBDs are colored in wheat, the two other NTDs are in pink, and the rest of the S1 and S2 subunits are in green.
Identifying the biophysical determinants of mutational tolerability. (A-B) The relationship between relative solvent accessibility (RSA) and the mutational tolerability is shown for (A) RBD and (B) NTD. The deep mutational scanning data on RBD expression was from a previous study42. (C-D) The relationship between sequence conservation among 27 sarbecovirus strains and (C) the mutational tolerability, or (D) RSA of each NTD residue is shown. of each NTD residue is shown. (A-E) Each data point represents one residue. The Spearman’s rank correlation coefficient (ρ) is indicated. (F) The mutational tolerability of residues within the cross-neutralizing NTD antibody epitopes (C1520, C1717, C1791)16 is compared to that within the antigenic supersite14 using a violin plot. Each data point represents one residue. P-values were computed by a two-tailed t-test. (G) The mutational tolerability of each NTD residue is projected on one NTD of the S trimer structure (PDB 6ZGE44 and PDB 7B6245). Red indicates residues with higher mutational tolerability, while blue indicates residues with lower mutational tolerability. Residues with insufficient data to calculate mutational tolerability are colored in grey. Two residues of interest, namely S50 and G232, are indicated. RBDs are colored in wheat, the two other NTDs are in pink, and the rest of the S1 and S2 subunits are in green.
The two outliers, residues G232 and S50, displayed good mutational tolerance despite being close to RBD and S2, respectively. Two NTD mutations, G232E and S50Q, at the interdomain contact enhanced S protein expression without changing its antigenicity. The team found that G232E and S50Q in the NTD boost the expression rate of the S protein without modifying its melting temperature (Tm), similar to several previously reported mutations in the S2 unit.
Notably, G232E and S50Q were only two of numerous mutations that increase S protein expression, as per the deep mutational scanning data. Consequently, the current findings imply that mutations in NTD could offer a complementary method, even if the majority of research on S-based immunogen design concentrates on the S2 and RBD mutations.
Collectively, the present findings have significant repercussions for comprehending NTD dynamics and S-based immunogen synthesis.
Conclusions
According to the current research using deep mutational scanning, numerous NTD mutations at buried residues did not impact the expression of the SARS-CoV-2 S protein. Meanwhile, the closer an NTD mutation was to RBD/S2, the more probably it was damaging to the S protein expression. These findings suggest that the structural stability at the NTD-S2 and NTD-RBD interfaces was more relevant for optimal S protein expression than the NTD's folding stability.
The present results partially describe why the N1 to N5 loops, which were distant from the NTD-RBD/S2 interfaces and comprise the NTD antigenic supersite, differ notably between SARS-CoV-2 variants and sarbovirus strains. In addition to potentially increasing the efficacy of messenger ribonucleic acid (mRNA) vaccines, the authors stated that designing high expressing S protein might reduce the cost of producing recombinant SARS-CoV-2 vaccine.
Overall, the present study contributes to the knowledge of the SARS-CoV-2 NTD's biophysical limitations and offers priceless information regarding S-based immunogen development. The researchers stated that future works should methodically examine how various NTD mutations influence SARS-CoV-2 replication fitness to elucidate the biophysical limits of NTD completely.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Probing the biophysical constraints of SARS-CoV-2 spike N-terminal domain using deep mutational scanning; Wenhao O. Ouyang, Timothy J.C. Tan, Ruipeng Lei, Ge Song, Collin Kieffer, Raiees Andrabi, Kenneth A Matreyek, Nicholas C. Wu. bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.06.20.496903, https://www.biorxiv.org/content/10.1101/2022.06.20.496903v1
- Peer reviewed and published scientific report.
Ouyang, Wenhao O., Timothy J.C. Tan, Ruipeng Lei, Ge Song, Collin Kieffer, Raiees Andrabi, Kenneth A. Matreyek, and Nicholas C. Wu. 2022. “Probing the Biophysical Constraints of SARS-CoV-2 Spike N-Terminal Domain Using Deep Mutational Scanning.” Science Advances 8 (47). https://doi.org/10.1126/sciadv.add7221. https://www.science.org/doi/10.1126/sciadv.add7221.