Human milk gives passive immunity to breastfed infants, primarily in the form of immunoglobulin A (IgA), against infections. While IgA, a component of mucosal immunity, is the first line of defense, IgG is abundant in serum and mounts the secondary immune response that sustains for several months.
Thus, maternal vaccination against COVID-19 likely offers protection to the breastfed infant. Since specific human milk antibodies decrease over time, it is crucial to evaluate whether booster shots restore human milk antibody levels, potentially prolonging an infant's passive immunity.
About the study
In the prospective follow-up study, researchers recruited 26 lactating women who had received a messenger ribonucleic acid (mRNA)-based COVID-19 booster. All women had received BNT162b2 except one who received the mRNA-1273 booster shot.
During their primary vaccination, four and nine women among homologous booster vaccination recipients (total of 13) had received BNT162b2 and mRNA-1273 vaccines, respectively. Among 13 women who were heterologous booster vaccination recipients, eight and five women received AZD1222 and Ad26.COV2.S vaccines, respectively.
Both cohorts of women had been breastfeeding for an average time of 6.6 months and had no SARS-CoV-2-specific antibodies in their serum before vaccination. The researchers collected human milk samples in sterile polypropylene tubes on days three, five, seven, nine, 11, 13, and 15 after booster vaccination and one before vaccination from all participating women. They stored all samples at −80 °C till analysis.
Next, they measured SARS-CoV-2-specific IgA and IgG antibodies in human milk using an enzyme-linked immunosorbent assay (ELISA). Finally, the researchers assessed the total antibody response up to 15 days after the booster vaccination, represented as the area under the curve (AUC).
Study findings
The researchers analyzed 199 milk samples for the present study. They noted that before boosting, the average SARS-CoV-2-specific IgA antibodies in milk were comparable for both groups. In the homologous booster vaccination cohort, all had IgA in their milk at a time point. However, detectable IgA levels persisted in the milk of only 12/13 participants throughout the follow-up period. Overall higher levels of SARS-CoV-2-specific IgA and IgG in their human milk likely was due to overall higher pre-booster levels.
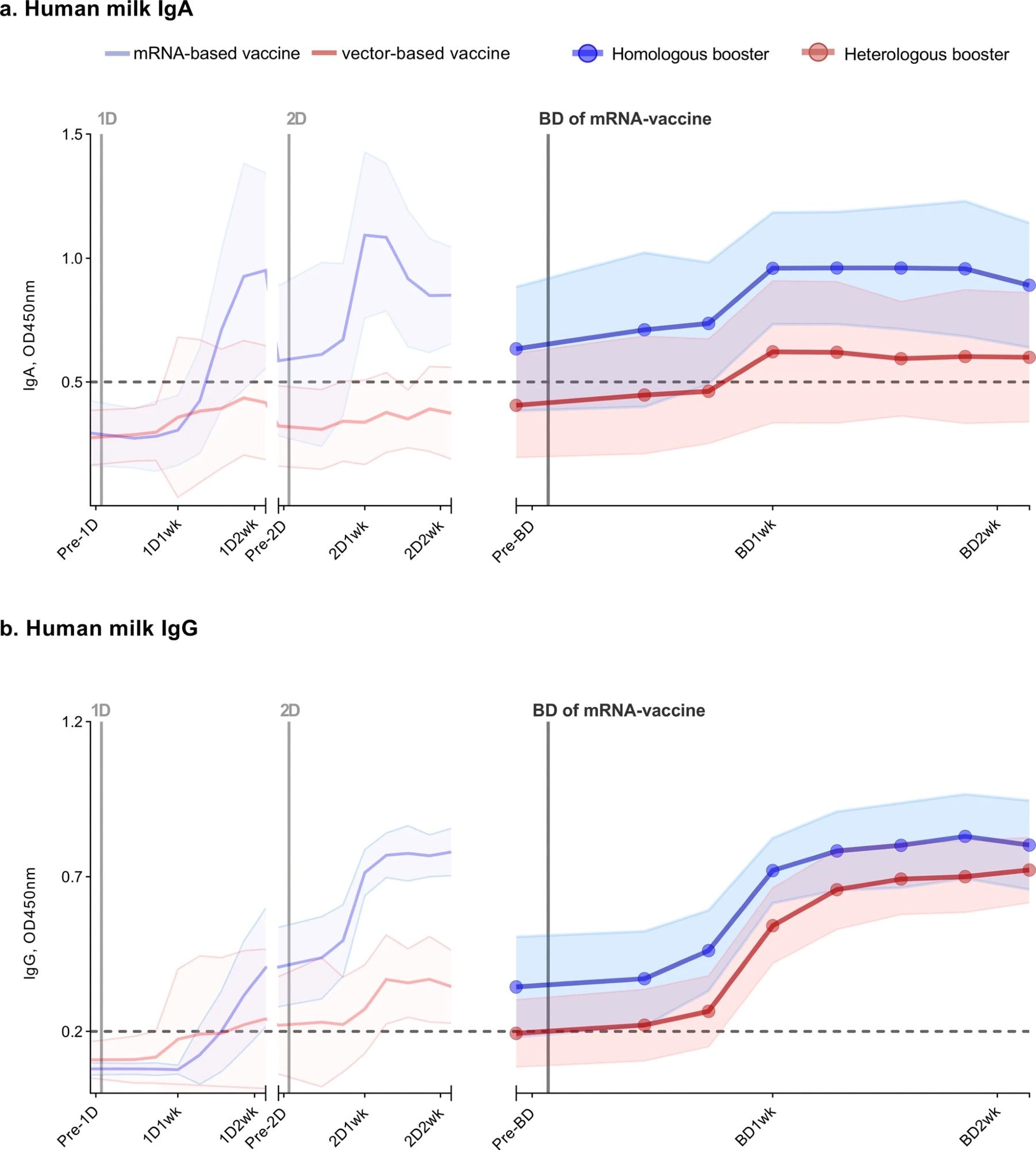 Antibody response in human milk of 26 lactating women vaccinated against COVID-19. SARS-CoV-2-specific immunoglobulin A (a) and immunoglobulin G (b) in human milk were measured using an ELISA with the SARS-CoV-2 spike protein. Participants either received a primary series with an mRNA vaccine (2 × mRNA-1273 or 2 × BNT162b2), subsequently following a homologous boosting schedule (n = 13), or a vector-based vaccine (2 × AZD1222 or 1 × Ad26.COV2.S) and thus following a heterologous boosting schedule (n = 13). The left part of both graphs (from pre-1D to 2D2wk), is already described in our previous study. The time between the first dose and booster dose in the homologous group was, on average, 338 days (BNT162b2) and 262 days (mRNA-1273), and in the heterologous group, 234 days (Ad26.COV2.S) and 285 days (AZD1222). Analyzed samples were collected prior to a vaccine dose, 3 days, 5 days, 7 days, 9 days, 11 days, 13 days, and 15 days after a vaccine dose. 1D, first vaccine dose; 2D, second vaccine dose; BD, mRNA-vaccine booster vaccine; Pre, one day prior to; 1wk, one week after; 2wk, two weeks after vaccine dose. Data points represent the mean of all measurements per group; the shaded area around the mean represents the standard deviation. Cutoff values are indicated with dashed lines. OD450nm, optical density 450 nm.
Antibody response in human milk of 26 lactating women vaccinated against COVID-19. SARS-CoV-2-specific immunoglobulin A (a) and immunoglobulin G (b) in human milk were measured using an ELISA with the SARS-CoV-2 spike protein. Participants either received a primary series with an mRNA vaccine (2 × mRNA-1273 or 2 × BNT162b2), subsequently following a homologous boosting schedule (n = 13), or a vector-based vaccine (2 × AZD1222 or 1 × Ad26.COV2.S) and thus following a heterologous boosting schedule (n = 13). The left part of both graphs (from pre-1D to 2D2wk), is already described in our previous study. The time between the first dose and booster dose in the homologous group was, on average, 338 days (BNT162b2) and 262 days (mRNA-1273), and in the heterologous group, 234 days (Ad26.COV2.S) and 285 days (AZD1222). Analyzed samples were collected prior to a vaccine dose, 3 days, 5 days, 7 days, 9 days, 11 days, 13 days, and 15 days after a vaccine dose. 1D, first vaccine dose; 2D, second vaccine dose; BD, mRNA-vaccine booster vaccine; Pre, one day prior to; 1wk, one week after; 2wk, two weeks after vaccine dose. Data points represent the mean of all measurements per group; the shaded area around the mean represents the standard deviation. Cutoff values are indicated with dashed lines. OD450nm, optical density 450 nm.
Conversely, in the heterologous booster vaccination cohort, nine of 13 participants had substantial IgA levels in their milk at least once during the follow-up period. In addition, all the participants had SARS-CoV-2-specific IgG in their milk throughout the follow-up period, irrespective of the vaccination schedule they followed.
After vaccination, IgA and IgG antibodies in human milk increased after 15 days. From pre-booster to day 15, the average IgG antibodies in milk gradually increased. On the contrary, average IgA levels in milk stagnated between seven and 15 days following vaccination, showing a slight decline. The vaccine type used for the primary vaccine series did not affect the elicited antibody response following boosting.
Conclusions
Consistent with findings in blood serum, SARS-CoV-2-specific antibodies declined several months after a primary COVID-19 vaccination in human milk. Boosting with an mRNA-based vaccine increased the magnitude of these antibodies in human milk, irrespective of the schedule of primary vaccination series (homo- or heterologous). However, as expected, participants in the homologous booster vaccination cohort, who started from higher pre-booster SARS-CoV-2-specific antibody levels, consistently had more antibodies in their milk after boosting.
COVID-19 vaccines trigger a heightened systemic response, so IgG levels steadily increased in human milk post-boosting. In addition, IgG appeared earlier than IgA in human milk. Comparatively, the IgA response was modest, with more inter-individual variation post-boosting. Nevertheless, an mRNA-based booster shot induced a significant antibody response in human milk independent of the initial vaccine type, which, in turn, would confer protection against COVID-19 to breastfed infants. Overall, these findings favor recommendations for giving mRNA-vaccine boosters to lactating women.