A breakthrough sweat-sensing patch reveals your body’s hidden stress chemistry, separating exercise from emotional strain and using AI to forecast anxiety before you even feel it.

Study: Stressomic: A wearable microfluidic biosensor for dynamic profiling of multiple stress hormones in sweat
In a recent study published in the journal Science Advances, a group of researchers reported and validated Stressomic, a wearable multiplexed microfluidic biosensor that performs time-stamped, sequential sampling at ≈6-minute intervals to quantify cortisol (CORT), epinephrine (EPI), and norepinephrine (NE) in sweat. This work enables on-body detection of EPI and NE in human sweat alongside cortisol.
Background
Many adults report feeling extreme stress, a state linked to cardiovascular disease and depression, and mental-health encounters are among the most common reasons for primary-care visits. Yet most people still gauge stress with surveys or fitness-band heart-rate readouts that cannot separate a joyful adrenaline rush from harmful, lingering tension. Biochemists have long known that CORT, EPI, and NE sketch a molecular timeline of stress, but measuring them has required needles and laboratories. A body-friendly sensor able to follow all three molecules could reveal hidden stress patterns, personalize mental-health care, and guide workplace safety. Further studies must refine accuracy across diverse populations.
About the study
Engineers fabricated the skin-conformal device, Stressomic, on a flexible printed circuit board (FPCB) carrying gold nanodendrite (AuND)-decorated laser-engraved graphene (LEG) working electrodes (WEs), a silver/silver-chloride reference electrode (RE), and integrated temperature, pH, and ionic-strength sensors. A multilayer microfluidic network containing capillary burst valves (CBVs) sequentially directed iontophoretically generated sweat over five reagent chambers. In each chamber, methylene-blue (MB)-labeled antigen competitors mixed with incoming fluid and competed with endogenous CORT, EPI, and NE for antibody sites. Square wave voltammetry (SWV) recorded the reduction currents, while a timer electrode time-stamped each filling event to normalize for inter-individual sweat-rate differences. Device biocompatibility was verified with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays and a 21-day guinea-pig skin-sensitivity protocol.
Analytical performance was established in buffer using differential pulse voltammetry (DPV), electrochemical impedance spectroscopy (EIS), and enzyme-linked immunosorbent assay (ELISA) cross-validation. Human evaluation enrolled healthy volunteers who completed high-intensity interval training (HIIT), viewed negative International Affective Picture System (IAPS) images, or followed a supplement protocol (taurine 1000 mg and a theanine-magnesium formulation [Theanine Serene with Relora]). Sweat was profiled every six minutes for 40 minutes via CBV-sequenced chambers, and affect was captured with the Positive and Negative Affect Schedule (PANAS) and State-Trait Anxiety Inventory (STAI), while all protocols followed review board approval.
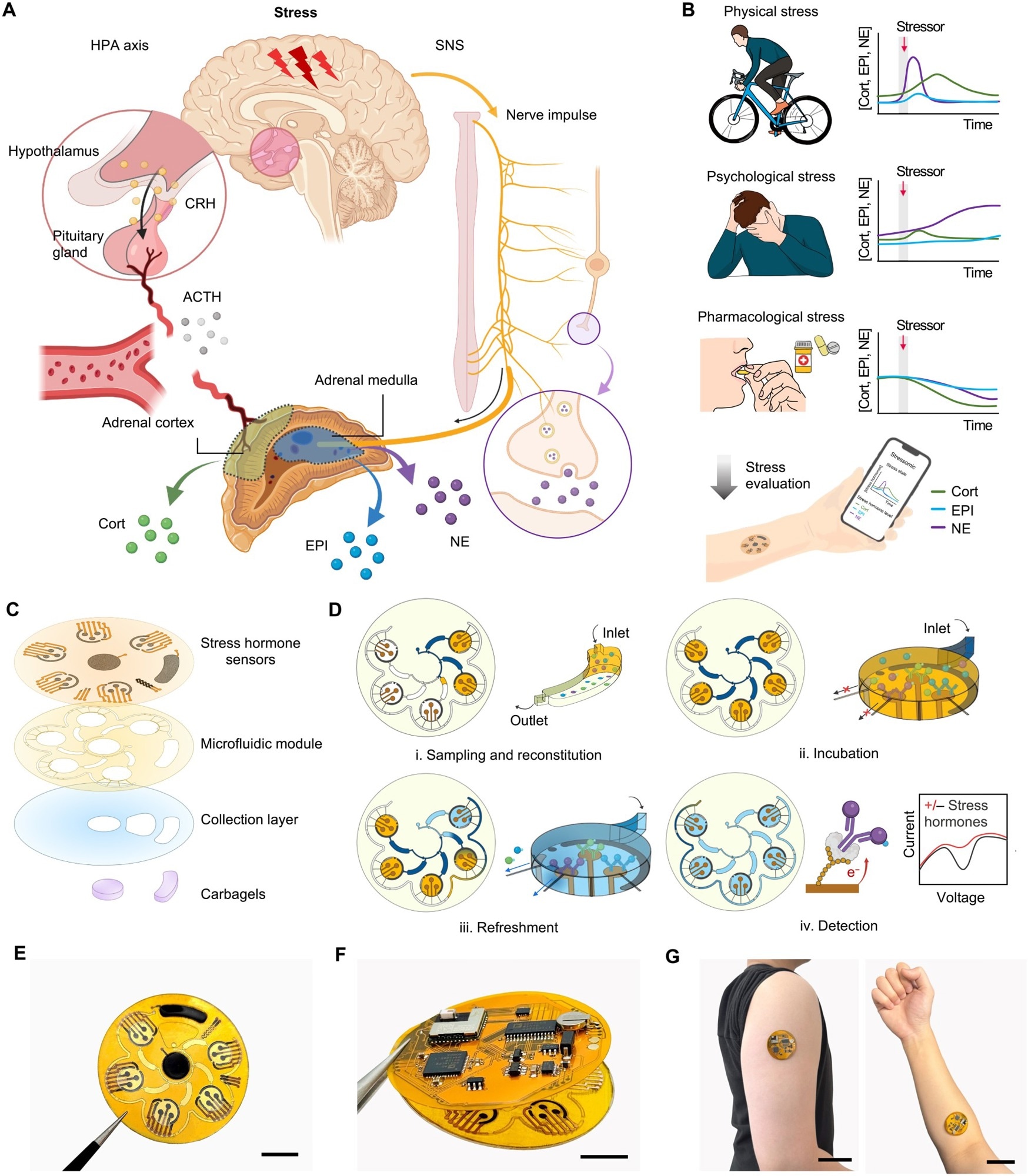
Overview of Stressomic, a multiplexed wearable stress hormone monitoring system. (A) Schematic illustration of the body’s stress response pathways. The HPA axis triggers cortisol (Cort) release via corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), while the SNS stimulates the adrenal medulla to release epinephrine (EPI) and norepinephrine (NE) into the bloodstream and secrete NE at nerve terminals to regulate local tissue responses. (B) Representative profiles of Cort, EPI, and NE levels under physical stress (HIIT), emotional stress (IAPS), and after stress modulation (supplementation). These data illustrate example trends rather than continuous hormone dynamics. (C) Design of Stressomic, consisting of three hormone biosensors, a microfluidic module for automated sequential sweat sampling, reconstitution, and reagent delivery, a collection layer that facilitates sweat sampling and accumulation, and a pair of carbachol-loaded hydrogels for on-demand sweat induction. (D) Workflow of the microfluidic system for stress hormone detection: (i) Sweat fills reagent and detection chambers, reconstituting analytes in buffer with redox-labeled competitors; (ii) Analytes incubate and compete for binding at electrodes in detection chambers; (iii) Unbound molecules are flushed to preserve sensor accuracy during automated refresh cycles; (iv) Electrochemical detection quantifies hormones by measuring redox signals, enabling sensitive, real-time monitoring. (E to G) Photos of Stressomic, including the microfluidic sensor patch (E), fully integrated wireless system (F), and a system worn on the upper arm and forearm (G). Scale bars, 1 cm (E and F) and 5 cm (G).
Study results
Electrode morphology studies revealed a porous LEG scaffold blanketed by high-surface-area AuNDs, a configuration that lowered charge-transfer resistance and amplified redox signals. SWV produced log-linear calibration curves spanning 0–100 ng mL⁻¹ (CORT) and 0–100 pg mL⁻¹ (catecholamines), with limits of detection of 2.70 ng mL⁻¹, 2.73 pg mL⁻¹, and 9.14 pg mL⁻¹, respectively. Sweat-derived currents matched ELISA readings in sweat (n = 21) and correlated with serum (n = 74). The CBV architecture filled each of five chambers in six-minute increments, and the timer electrode confirmed flow independence across sweat rates from 0.5 to 3 μL min⁻¹. Under cyclic bending to a 1.5-cm radius, signals showed minimal variation, supporting on-body accuracy.
During HIIT, the wearable registered a clear CORT rise within the first ten minutes, a modest NE surge, and transient EPI traces; values returned toward baseline by 40 minutes. Psychological stress induced by IAPS images left CORT statistically unchanged yet produced a rise in NE (with EPI largely unchanged) that paralleled elevated STAI scores. Supplement ingestion drove progressive CORT decline and a small NE decrease; EPI remained relatively stable, and questionnaire scores did not change significantly, suggesting baseline neuroendocrine modulation rather than acute relief.
Random forest (RF) models trained on the first 20 minutes of hormone data predicted negative affect, positive affect, and state anxiety with accuracies of 62%, 54%, and 86%, respectively. SHapley Additive exPlanations (SHAP) showed that CORT dominated negative-affect classification, while NE and EPI contributed complementary information to anxiety prediction; no single biomarker alone explained the model’s decisions.
AuND deposition lowered resistance, protein-A/G attachment raised it, and 6-mercapto-1-hexanol (MCH) blocking stabilized signals, confirming consistent chemistry. The microfluidic layout maintained sensitivity despite rapid flow by enabling prolonged incubation via a burst-pressure gradient. Signal stability was maintained over the test period, demonstrating stable performance during on-body tests.
Across all experiments, inter-individual variability proved substantial, as some participants exhibited rapid hypothalamic-pituitary-adrenal (HPA) axis quenching, whereas others maintained elevated sympathetic nervous system (SNS) output well into recovery. The FPCB, Bluetooth Low Energy (BLE) telemetry, and custom mobile application streamed calibrated data in real time; on-body tests also interfaced with a laptop.
Conclusions
To summarize, the study demonstrates that a flexible FPCB-integrated microfluidic biosensor performs time-resolved, sequential quantification (approximately 6-minute resolution) of the three principal stress hormones in sweat with low-picomolar (catecholamines) to nanomolar (cortisol) sensitivity.
The technology distinguished physical exertion from psychological strain, captured the hormone-dampening effect of a dietary supplement, and predicted self-reported anxiety using machine learning powered by simultaneous CORT, EPI, and NE data. By delivering laboratory-grade analytics through a patch that streams results over BLE, the platform paves the way for personalized stress dashboards, early detection of maladaptive responses, and objective evaluation of mental-health interventions in workplaces, clinics, athletic arenas, and daily life.
The authors note batch-to-batch variability and recommend process standardization and batch-specific calibration in future iterations.
Journal reference:
- Tu, J., Yeom, J., Ulloa, J. C., Solomon, S. A., Min, J., Heng, W., Kim, G., Dao, J., Vemu, R., Pang, M., Wang, C., Kim, D.-H., & Gao, W. (2025). Stressomic: A wearable microfluidic biosensor for dynamic profiling of multiple stress hormones in sweat. Sci. Adv. 11(32). DOI:10.1126/sciadv.adx6491, https://www.science.org/doi/10.1126/sciadv.adx6491