The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been one of the greatest public health challenges worldwide. The World Health Organization (WHO) reports more than 254 million cases of SARS-CoV-2 infection and over 5.1 million deaths since the beginning of the pandemic.
SARS-CoV-1 and SARS-CoV-2 have similar sequences, with their shared primary entry receptor being the angiotensin-converting enzyme 2 (ACE2) receptor. Several studies regarding other host factors that can influence the spike binding of SARS-CoV-2 are limited.
Researchers from the University of Sydney recently conducted a study published on the preprint server bioRxiv* to identify novel host factors affecting the cellular interactions of the SARS-CoV-2 spike protein using whole-genome clustered regularly spaced short palindromic repeats (CRISPR) activation. A toll-like receptor (TLR)-related cell surface receptor known as leucine-rich repeat-containing protein 15 (LRRC15) was identified in three whole-genome screens as a promoter of SARS-CoV-2 spike binding.
Flow cytometry, immunoprecipitation, and confocal microscopy confirmed that LRRC15 forms a cell surface complex with the virus but did not support infection. LRRC15 is not an entry receptor for SARS-CoV-2; however, its ectopic expression inhibits SARS-CoV-2 pseudovirus infection and reduces live SARS-CoV-2 infection.
High-throughput screening for SARS-CoV-2 spike binding assay identifies LRRC15
Earlier studies have found ACE-2 as the primary receptor for the SARS-CoV-2 spike protein. The researchers of the current study used CRISPR activation screening to identify other host factors influencing the spike488 protein interaction.
Flow cytometry showed that ACE2 complementary deoxyribonucleic acid (cDNA) HEK293TACE2 has a high affinity to spike488 as compared to wild-type HEK293T cells. Later, three single guide ribonucleic acid (sgRNA) clones of HEK293T-CRISPRa also showed similar results as cDNA overexpression and confirmed that CRISPRa induced ACE2 expression benefits spike488 binding.
The researchers collected genomic DNA from unselected or spike488-selected cells and the abundance of sgRNA was quantified by sequencing. The data were subsequently analyzed using the MAGeCK analysis platform and plotted with MAGeCKFlute. Transmembrane protein LRRC15 was a top hit at an FDR cut-off of 0.25, followed by the SARS-CoV-2 entry receptor ACE2.
LRRC15 suppresses spike-mediated entry and infection of SARS-CoV-2
To confirm the role of LRRC15 in SARS-CoV-2 spike binding, the researchers transfected LRRC15-GFP cDNA into HEK293T cells and analyzed spike647 binding through flow cytometry. LRRC15 was found to have two isoforms of LRRC15_1 and LRRC15_2, both of which showed strong spike binding. No spike647 binding was observed in cells transfected with a green fluorescent protein (GFP).
The study also showed that LRRC15 expression promotes spike binding in HEK293T-ACE2-TMPRSS2 cells and that both isoforms of LRRC15 colocalized with spike647.
To confirm the interaction between the SARS-CoV-2 spike protein and LRRC15, the spike was added to LRRC15-expressing cells, followed by the immunoprecipitation of LRRC15. In the case of both LRRC15_1 or LRRC15_2, the spike protein was co-immunoprecipitated in the eluate.
Taken together, these findings demonstrate that expression of LRRC15 can promote SARS-CoV-2 spike binding to HEK293T cells, where LRRC15 enhances the spike interactions in the presence of ACE2 and TMPRSS2. The researchers also evaluated the role of LRRC15 as an entry receptor for SARS-CoV-2 and revealed that it does not serve as an entry receptor; however, LRRC15 can suppress spike-mediated entry and infection of SARS-CoV-2.
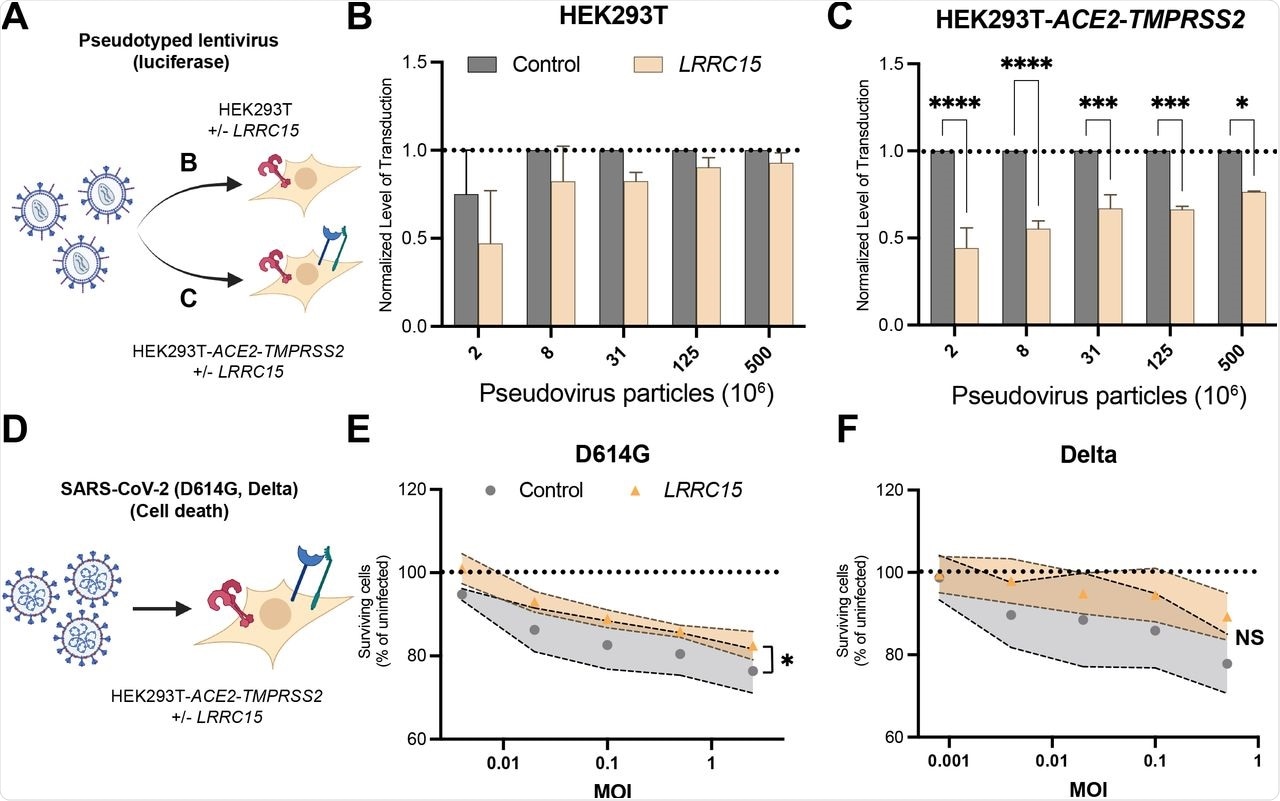 (A) Luciferase assay for quantification of SARS-CoV-2 pseudovirus infection in (B) WT HEK293T (n=4) and (C) HEK293T-ACE2-TMPRSS2(n=3). Cells were transfected with plasmid encoding LRRC15 transcript 1 or empty vector as a control. Luminescence for LRRC15 cells were normalized to Control cells. Significance was determined by two-way ANOVA, Sidak multiple comparison test; ****p<0.0001,***p<0.001,**p<0.01,*p<0.05.
(A) Luciferase assay for quantification of SARS-CoV-2 pseudovirus infection in (B) WT HEK293T (n=4) and (C) HEK293T-ACE2-TMPRSS2(n=3). Cells were transfected with plasmid encoding LRRC15 transcript 1 or empty vector as a control. Luminescence for LRRC15 cells were normalized to Control cells. Significance was determined by two-way ANOVA, Sidak multiple comparison test; ****p<0.0001,***p<0.001,**p<0.01,*p<0.05.
(D) Cell death assay for quantification of D614G SARS-CoV-2 live virus infection in HEK293T-ACE2-TMPRSS2 cells. Cell death was determined via nuclei counts 48 hours after addition of virus.
(E,F) Quantification of cell survival after incubation with (E) D614G (n=4) and (F) Delta (n=3) SARS-CoV-2 live virus. Significance was determined by two-way ANOVA, *p<0.05.
LRRC15 regulates fibrosis
The current study used the COVID-19 Cell Atlas data set to confirm LRRC15 expression in multiple lymphatic vessels, placenta decidua stromal cells, and fibroblasts from the skin, prostate, and lungs. LRRC15 expression was subsequently confirmed in lung fibroblasts and neuronal cells in non-SARS-CoV-2-infected people.
Similar results were observed in other COVID-19 patients’ single-cell RNA sequencing data. These findings support the researchers’ in vitro observations, which show that LRRC15 acts as an innate immune barrier and does not facilitate SARS-CoV-2 infection.
In vitro studies of COVID-19 patients’ lungs showed that collagen-producing fibroblasts express LRRC15, which may regulate lung fibrosis. Based on all these findings, the researchers concluded that the TLR-related receptor LRRC15 is a regulator of infection as well as fibrosis, thus controlling the outcomes of SARS-CoV-2 infection and “long-haul” COVID-19.
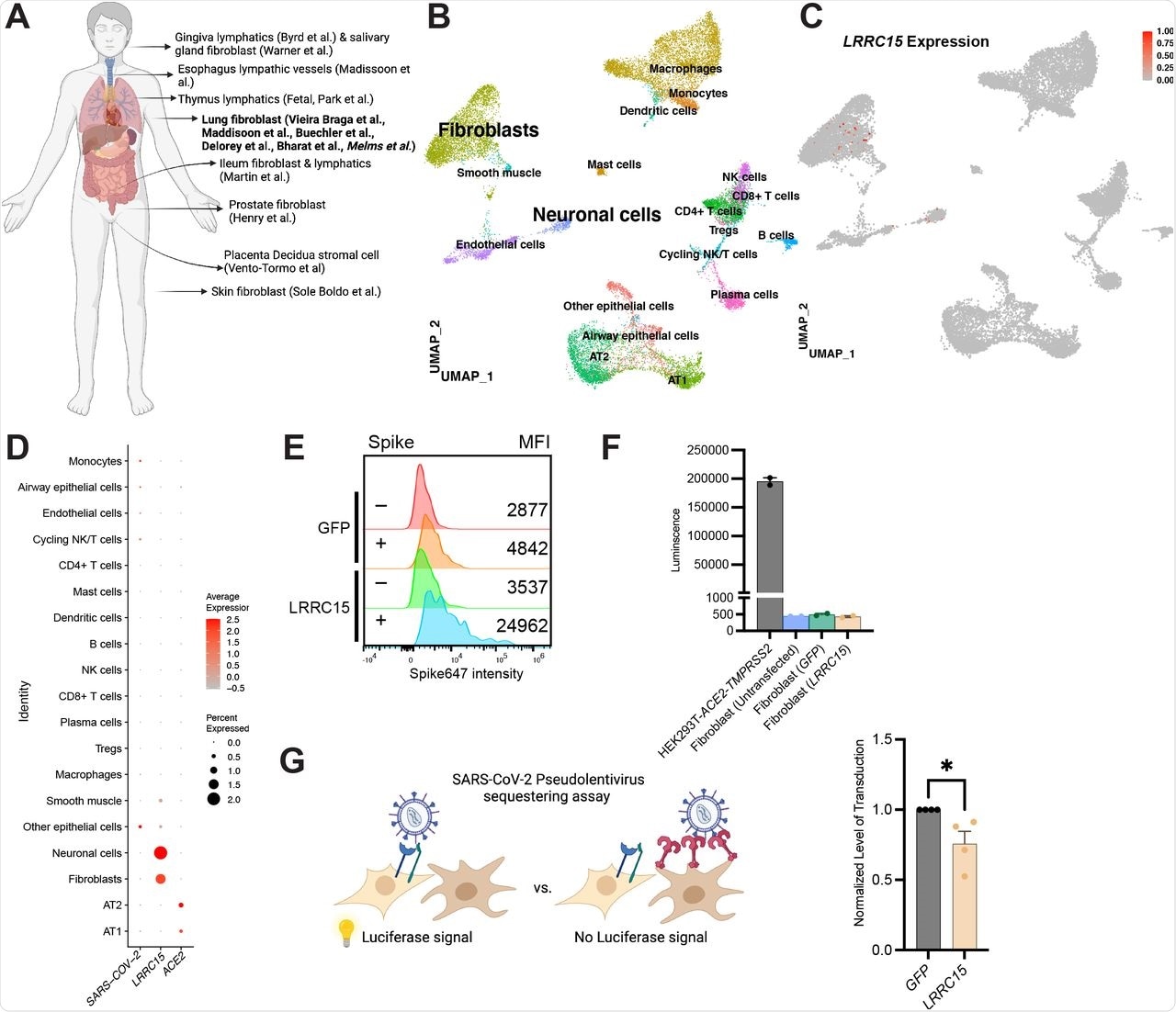 (A) Overview of cell types expressing LRRC15 from existing single cell RNA-sequencing datasets.
(A) Overview of cell types expressing LRRC15 from existing single cell RNA-sequencing datasets.
(B) UMAP plot of lung single nucleus RNAseq dataset (Melms et al).
(C) Feature plot and (D) Dotplot shows LRRC15 is expressed in fibroblasts and neuronal cells. Expression of LRRC15 in fibroblasts is also observed in fibroblasts of separate studies.
(E) Fibroblasts have intrinsic spike binding ability that can be further enhanced by LRRC15 overexpression. Fibroblasts were transfected with empty vector control or LRRC15 cDNA, and spike binding capacity was quantified via flow cytometry. MFI = Mean Fluorescence Intensity.
(F) Fibroblasts do not have innate tropism for SARS-CoV-2 and overexpression of LRRC15 does not mediate infection. Untransfected, GFP and LRRC15-GFP transfected fibroblasts were transduced with 5×108SARS-CoV-2 pseudovirus particles for 24 hours before quantification via luciferase assay. Transduction efficiency (luciferase luminescence) was compared to permissive cell line HEK293T-ACE2-TMPRSS2.
(G) LRRC15 expressing fibroblasts reduced SARS-CoV-2 pseudovirus transduction in HEK293T-ACE2-TMPRSS2. Luminescence of LRRC15+ co-culture was normalized to control GFP co-culture, and significance was determined by Mann-Whitney One-tailed test, *p<0.05.
“Further investigation into how LRRC15 contributes to SARS-CoV-2 349 pathology will help us better understand and treat this and future pandemics.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Loo L., Waller M. A., Cole, A. J., et al. (2021). LRRC15 suppresses SATS-CoV-2 infection and controls collagen production. bioRxiv. doi:10.1101/2021.11.09.467981. https://www.biorxiv.org/content/10.1101/2021.11.09.467981v1.
- Peer reviewed and published scientific report.
Loo, Lipin, Matthew A. Waller, Cesar L. Moreno, Alexander J. Cole, Alberto Ospina Stella, Oltin-Tiberiu Pop, Ann-Kristin Jochum, et al. 2023. “Fibroblast-Expressed LRRC15 Is a Receptor for SARS-CoV-2 Spike and Controls Antiviral and Antifibrotic Transcriptional Programs.” Edited by Ken Cadwell. PLOS Biology 21 (2): e3001967. https://doi.org/10.1371/journal.pbio.3001967. https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3001967.