When the respiratory illness SARS (Severe Acute Respiratory Syndrome) emerged in 2003, it killed at least 775 people before it was contained. Nine years later, MERS (Middle East Respiratory Syndrome) began circulating in the human population—and has gone on to have a 36 percent case fatality rate.
These diseases are species of coronaviruses, rapidly evolving pathogens that can “spill over” from animal populations to humans.

Key authors of the research included (left to right) The Scripps Research Institute’s Graduate Student Christopher Cottrell, Associate Professor Andrew Ward and Research Associate Robert Kirchdoerfer.
“We need to be prepared for these viruses,” said Andrew Ward, associate professor at The Scripps Research Institute (TSRI).
Now Ward and his colleagues at TSRI, Dartmouth and the National Institutes of Health (NIH) have solved the structure of a key protein in HKU1, a coronavirus identified in Hong Kong in 2005 and highly related to SARS and MERS. They believe their findings will guide future treatments for this family of viruses.
“This is really the ground floor,” said Ward, who co-led the study with Jason S. McLellan, assistant professor of biochemistry in the Geisel School of Medicine at Dartmouth College. “Once you have structures, you can actually start to go after different coronaviruses, like MERS and SARS.”
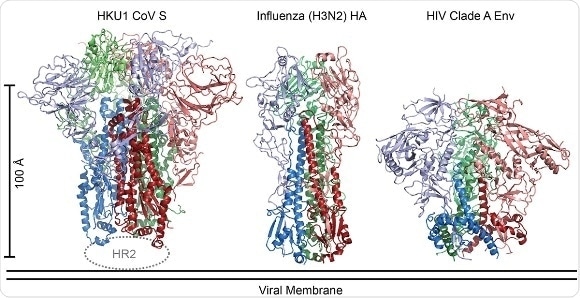
The coronavirus spike (CoV S) is the largest class I fusion protein known and displays structural similarities to the spike proteins of influenza virus and HIV. (Image courtesy of Robert Kirchdoerfer.)
The research was published today in the journal Nature.
The Virus’s Defenses
“Corona” means crown in Latin, and coronaviruses are named for their microscopic crowns of “spike” proteins.
At the base of each spike protein is the virus’s fusion machinery, which the virus uses to enter host cells. The structure of this machinery rarely changes between coronavirus species, which means antibodies found to target the structure could work against many viruses in this family.
The challenge for researchers has been to find antibodies that can actually reach the cell fusion machinery. Protecting this critical viral machinery is a layer of glycoproteins (proteins bound with sugars) that shields it from host antibodies’ attacks.
To help identify ways around the glycoprotein “shield,” scientists needed a map of the spike protein structure.
A New Roadmap
In the new study, Ward and his colleagues used an imaging technique called cryo-electron microscopy—in which samples are frozen and then imaged with an electron beam—to solve the spike protein’s 3D structure. The results show that HKU1 spike proteins have two interwoven “lobes” that crisscross over the fusion machinery and form a kind of inverted bell shape—a conformation not apparent in previous studies of only parts of the structure.
“That’s not something you would have predicted based on the previously solved coronavirus glycoprotein domains,” said TSRI Graduate Student Christopher Cottrell, who served as first author of the paper with TSRI Research Associate Robert Kirchdoerfer.
“Everything about this was surprising,” Kirchdoerfer added. “This is really one of the first pictures we have of a human coronavirus spike protein.”
The new structural image is also the first to hint at how the virus recognizes host cell receptors. It appears that the presence of human cell receptors can trigger the spike protein to shift its conformation and reveal its fusion machinery.
Although the exact mechanism behind the change is still unknown, Kirchdoerfer sees this phenomenon as a vulnerability that future therapies might exploit. “You might be able to induce that conformational change with an antibody,” he said.
The researchers hope to be ready for a future coronavirus spillover event. Thanks to new techniques in sample preparation and advances in cryo-electron microscopy automation and cameras, the recent project took months, rather than years, to complete.
“This is a nice demonstration of how we can leverage what we’ve done in different areas such as HIV and Ebola to rapidly solve structures of emerging pathogens,” said Ward.
The next step in this research will be to find human or animal antibodies that can target sites of vulnerability in the spike protein structure—revealing a way to get around the glycoprotein layer and neutralize the fusion machinery inside. Antibodies with this ability could be developed for therapeutics.