The modified vaccina virus Ankara (MVA) is an attenuated vaccinia virus that has been used as a vector in the already approved Adenovirus-MVA-based Ebola vaccine. A new preprint appearing on the bioRxiv* server reports the preclinical testing of a recombinant MVA (rMVA) candidate vaccine against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent that is causing the current coronavirus disease 2019 (COVID-19) pandemic. The MVA provides a vector platform for the rapid production of such vaccines.
Antibody responses in mice
This was proved to elicit specific antibodies against the virus in a mouse experiment. Both low and high doses (107 or 108 plaque-forming units (PFU), respectively), were used for intramuscular vaccination. Following the first or prime dose, specific IgG antibodies appeared in serum from 3/8 and 4/6 animals in the low-dose and high-dose groups, respectively. All showed high IgG antibody titers by day 21, after the second or booster dose had been given.
These antibodies also bound the S receptor-binding domain (RBD) as well, and were found in 7/8 and 100% of low-dose and high-dose mice, respectively, after the booster dose.
Neutralizing antibodies were also found in all serum samples after the booster dose, by two different assays, the plaque reduction neutralization test 50 (PRNT50) and a complete virus neutralization test (VNT50). These results were validated in a new high-throughput surrogate virus neutralization test for SARS CoV-2 (sVNT).
T cell responses in mice
The researchers also found that the vaccinated mice showed significant numbers of activated CD8 T cells after a single dose, in both low-dose and high-dose groups. After the booster dose, the magnitude of activation of the S-specific T cells increased. All activated cells expressed IFN-γand the majority also expressed TNF-α. The researchers also found activated CD4 T cells in both groups.
Protection against productive SARS-CoV-2 infection
The researchers next vaccinated mice and then exposed them intratracheally to the virus, at 2 weeks from the booster dose. These mice were euthanized four days later. While the control mice were found to be heavily infected, all the immunized mice had substantially lower levels of SARS-CoV-2 RNA. Infectious virus was also found at high levels in the control mice but not in vaccinated mice. This indicates that the vaccinated mice had developed immune responses that efficiently inhibited SARS-CoV-2 replication. The sera from these mice also contained specific neutralizing antibodies.
Implications
The researchers used the MVA viral vector, which replicates efficiently in DF-1 cells, making it suitable for rapid large-scale commercial vaccine production. Using their earlier experience with the Middle East respiratory syndrome coronavirus (MERS-CoV), they engineered the MVA to express the full-length SARS-CoV-2 spike protein in a stable manner when serially amplified.
The virus expressed the native S protein that was confirmed by a full range of characterization tests. The spike protein was moved through the host cell Golgi apparatus and localized at the cell surface. This suggests the MVA expresses a mature and properly folded spike protein.
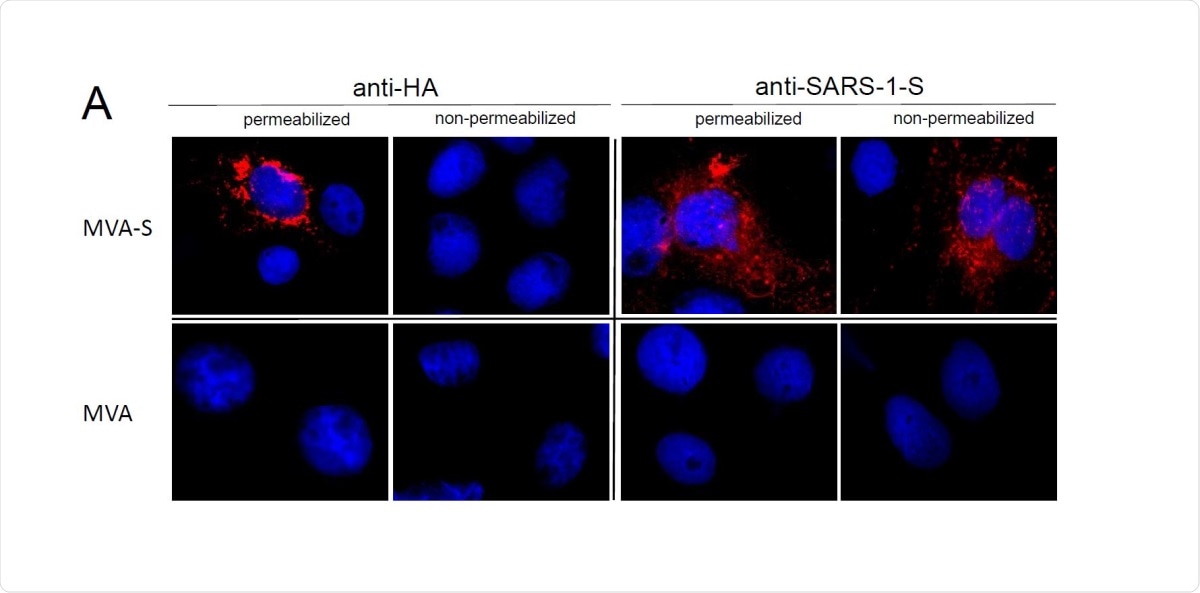
Synthesis of full-length Spike glycoprotein in MVA-SARS-2-S (MVA-S) infected cells. (A) Cells were infected at a multiplicity of infection of 0.5. MVA infected cells served as controls. Paraformaldehyde fixed cells were either permeabilized or non-permeabilized and probed with mouse monoclonal antibodies directed against the HAtag or the S protein of SARS-Cov-1 (SARS-1-S). Polyclonal goat anti-mouse secondary antibody was used for S-specific fluorescent staining (red). Cell nuclei were counterstained with DAPI (blue).
This spike antigen was shown to induce circulating specific IgG antibodies that neutralized the virus in cell culture, and also induced high levels of specific CD8 T cells. The neutralizing antibody titers were similar to those induced by current experimental vaccines.
The prime-boost regimen showed superior efficacy, in agreement with phase I clinical testing of the MVA MERS-S candidate vaccine earlier developed by the same researchers. There was no sign of antibody-dependent enhancement of disease in vaccinated animals.
The strong T cell response found following vaccination with this virus showed not only that this vaccine produces adaptive immunity. No infectious virus was recovered from the lung tissue of any vaccinated animals, in either the low-dose or high-dose group. This is important in view of the many studies that indicate the importance of a strong cell-mediated immune response in COVID-19 recovery, with a robust CD4 or CD8 T cells response to SARS-CoV-2 being linked to mild disease in such individuals.
The MVA-based SARS-CoV-2 spike-expressing candidate vaccine is thus both safe and immunogenic, in preclinical animal studies. Based on these results, the investigators have begun a phase I clinical trial on September 30, 2020. They hope that such vaccines will provide optimized immunity, across the spectrum of neutralizing activity as well as CD4 and CD8 T cells, against the SARS-CoV-2 infection, in multiple population groups.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Tscherne, A. et al. (2021). Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA SARS 2 S in preclinical vaccination. bioRxiv preprint. doi: https://doi.org/10.1101/2021.01.09.426032, https://www.biorxiv.org/content/10.1101/2021.01.09.426032v1
- Peer reviewed and published scientific report.
Tscherne, Alina, Jan Hendrik Schwarz, Cornelius Rohde, Alexandra Kupke, Georgia Kalodimou, Leonard Limpinsel, Nisreen M. A. Okba, et al. 2021. “Immunogenicity and Efficacy of the COVID-19 Candidate Vector Vaccine MVA-SARS-2-S in Preclinical Vaccination.” Proceedings of the National Academy of Sciences 118 (28). https://doi.org/10.1073/pnas.2026207118. https://www.pnas.org/doi/full/10.1073/pnas.2026207118.