Researchers in the United States and the UK have conducted a study showing that the rollout of vaccines to protect against coronavirus disease 2019 (COVID-19) is proving effective among California's general population.
The population-based surveillance study found that vaccination efforts are preventing infection with the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) under real-world conditions, despite the widespread circulation of variants of concern.
Working on behalf of the California COVID-19 Case-Control Study Team, the researchers found that among fully vaccinated individuals (the second dose received two weeks or more previously), protection against SARS-CoV-2 infection was 86%.
Partial protection was also evident following one vaccine dose and within the first two weeks of receiving a second dose, says the team from the University of California at Berkeley, California Department of Public Health in Richmond, and the London School of Hygiene and Tropical Medicine.
"Our findings indicate that vaccine rollout is preventing COVID-19 in the general population of California," writes Joseph Lewnard and colleagues.
However, the study also found that vaccine hesitancy presents a barrier to reaching the coverage levels required for herd immunity to be achieved.
The team says different communication and outreach approaches will be needed to address these barriers to vaccine uptake across communities.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.
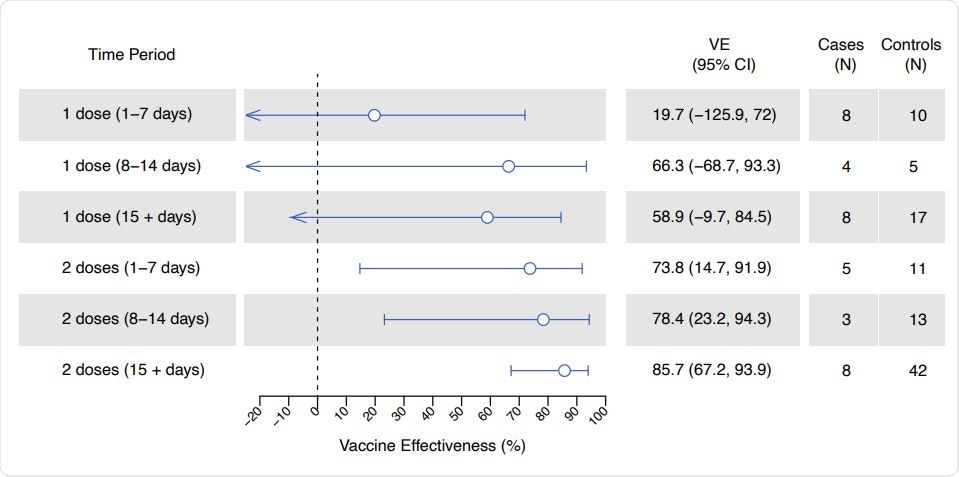
COVID-19 vaccine effectiveness, by doses received and time since last dose. Lines denote 95% confidence intervals, respectively, for estimates of vaccine effectiveness. Estimates were calculated via conditional logistic regression.
Studies of real-world vaccine effectiveness are required
Following demonstration of their efficacy in randomized controlled trials, the Pfizer-BioNtech BNT162b2 and Moderna mRNA1273 vaccines have been administered since December 2020. By the end of April 2021, the vaccines had been administered to 24% of California residents.
"Observational studies characterizing vaccine effectiveness are needed to understand performance under real-world conditions and to inform implementation programs," says Lewnard and the team.
To date, most studies of real-world vaccine effectiveness have focused on healthcare workers and other frontline staff. They have also been performed in settings where variants of concern were not yet accounting for a substantial proportion of SARS-CoV-2 infections.
However, as of March 2021, the B.1.1.7 and B.1.427/B.1.429 variants of concern that emerged in the UK and California, respectively, accounted for 69% of all SARS-CoV-2 isolates in California.
What did the researchers do?
To determine the effectiveness of the BNT162b2 and mRNA-1273 vaccines in the context of substantial circulation of these variants, the researchers conducted a case-control study of adult California residents with SARS-CoV-2 test results reported to the California Department of Public Health between 24th February and 7th April 2021.
Individuals who tested negative for SARS-CoV-2 infection (controls) were individually matched to SARS-CoV-2 positive individuals (cases) by age, sex, geographic region and the week of testing. A standardized questionnaire covering participants' demographics, symptoms, and vaccination status was administered via telephone-based interviews.
Individuals were considered fully vaccinated two weeks after having received a second dose of either vaccine.
To help identify vaccine uptake barriers that might inform future immunization strategies, unvaccinated individuals were also asked about their willingness to receive a vaccine once they became eligible.
What did the study find?
Among 325 cases, 23 (7%) and 13 (4%) reported receiving at least one dose of the BNT162b2 vaccine and mRNA-1273 vaccine, respectively, and eight cases (2%) were considered fully vaccinated.
Among 260 controls, 49 (19%) and 49 (19%) reported receiving at least one dose of BNT162b2 and mRNA-1273, respectively, and 42 (16%) were considered fully vaccinated.
Among the fully vaccinated individuals (at least two weeks since the second dose), vaccine effectiveness was 86%.
Vaccine effectiveness was 66% within the second week of receiving a first dose and 78% within the second week of receiving a second dose.
Rural-urban divides in vaccine willingness
Of 415 unvaccinated individuals, 39% of those residing in rural regions and 23% of those residing in urban regions indicated that they were unlikely or unsure about receiving a vaccine once they became eligible. By contrast, vaccine hesitancy did not significantly vary by age, sex, household income, or ethnicity.
The most commonly reported reasons for the hesitancy were fears over safety (24%) and side effects (26%).
"Vaccine rollout is preventing COVID-19 under real-world conditions"
The researchers say the ongoing vaccine rollout is preventing COVID-19 under real-world conditions in the general population.
Moreover, partial protection was identified before two weeks after receipt of the second dose, in agreement with previous estimates, they add.
"Importantly, though, our results indicate substantial protection despite the widespread circulation of the B.1.427/B.1.429 variants," writes Lewnard and colleagues.
However, vaccine hesitancy is presenting a barrier to reaching the coverage levels needed for herd immunity and "differing messaging and outreach strategies will thus be needed to address barriers to vaccine acceptance across communities," concludes the team.