Introduction
Serological evaluations have traditionally assisted physicians and scientists in assessing the efficacy of vaccines or the immune system to defend individuals against various infections. More recently, these types of studies have been used to estimate vaccine efficacy against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the coronavirus disease 2019 (COVID-19).
As of July 1, 2021, which is over 1.5 years since SARS-CoV-2 was originally detected in Wuhan, China in December 2019, COVID-19 has been responsible for over 182 million infections and almost 4 million deaths worldwide. The fight against COVID-19 has brought scientists from around the world together to develop effective vaccines against SARS-CoV-2, which have already been distributed in many countries worldwide.
To successfully bring the global population out of this pandemic, it is essential that the effects of these vaccines are widely studied and understood. However, the antibody response in COVID-19 patients and, especially in vaccinated subjects, remains largely unknown.
Evaluating S-RBD antibody levels post-vaccination
Recently, a group of Italian researchers evaluated the antibody levels in a large cohort to estimate the individual protection against the SARS-CoV-2. The individuals consisted of recipients of the COVID-19 mRNA BNT162b2 (Pfizer-BioNTech) vaccine, with or without previous SARS-CoV-2 infection, as well as recovered COVID-19 patients who did not receive the vaccination. The researchers investigated the demographic characteristics and anti-S protein- receptor-binding domain (S-RBD) immunoglobulin G (IgG) levels in the three groups and tabulated them in the paper.
This observational, single-center study was performed at the University Hospital P. Giaccone of Palermo, Italy between February 2021 to May 2021. Taken together, a total of 2,607 subjects were included in this study.
“To the best of our knowledge, this is the largest sample size study performed on the Italian population to date.”
Notable SARS-CoV-2 protein targets
The spike (S) and the nucleocapsid (N) proteins are the most potent immunogens among the SARS-CoV-2 proteins. The RBD of the S protein interacts directly with the host receptors and facilitates viral entry. Several COVID-19 serological diagnostic tests have been developed to detect antibodies that are against all or part of the N or S proteins of SARS-CoV-2.
“Although SARS-CoV-2 infection can induce the production of antibodies recognizing different viral antigens, antibodies directed against the RBD are the most relevant due to their neutralizing activity…It is noteworthy that the evaluation of antibodies against S-RBD IgG is the most important to assess the protection against SARS-CoV-2 infection due to their neutralizing activity.”
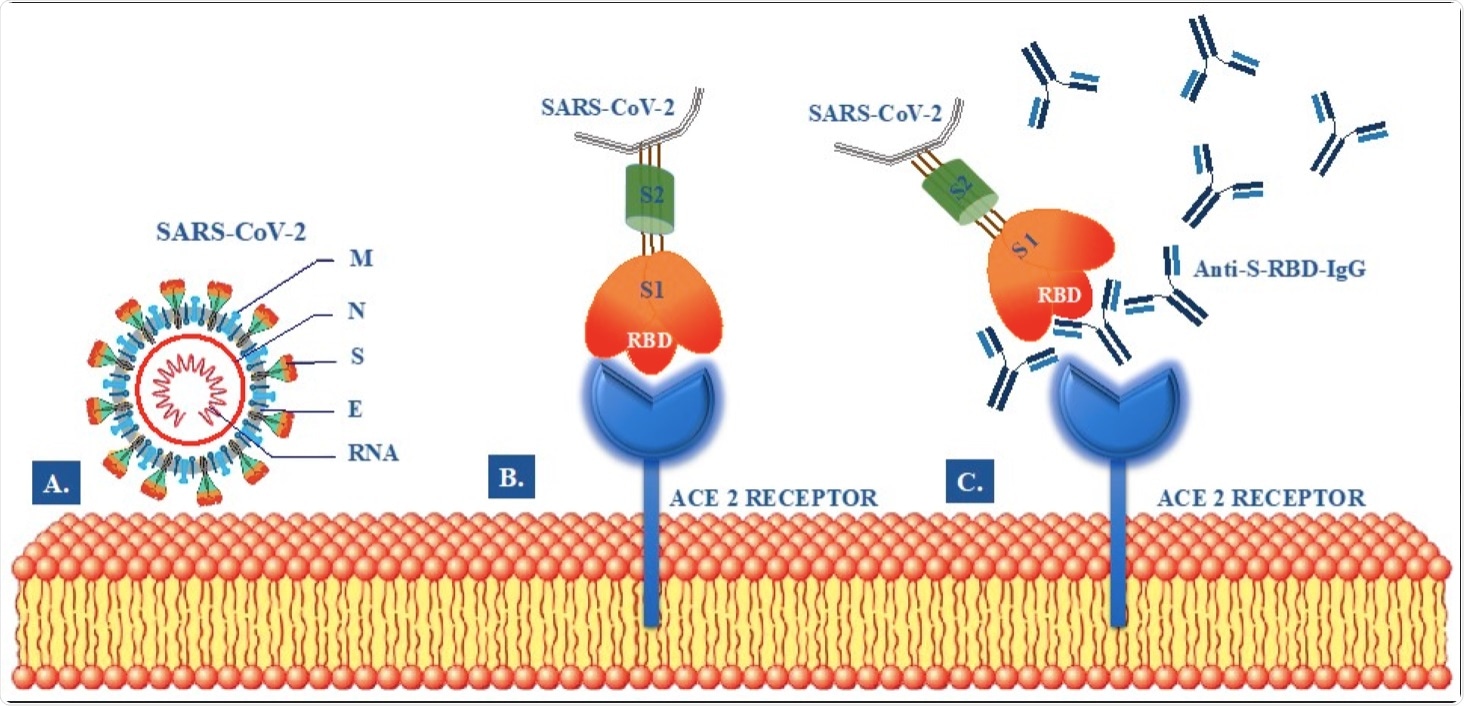 Schematic illustration of postulated mechanism modulating SARS-CoV-2 attachment, fusion and neutralizing activity of anti-S-RBD IgG SARS-CoV-2 antibodies. (A) Structural components SARS-CoV-2: Envelop Protein (E), Membrane glycoprotein (M), Nucleocapside protein (N), RiboNucleic Acid (RNA), Spike protein (S). (B) Binding of Receptor-Binding Domain (RBD) to the Angiotensin-converting enzyme 2 (ACE2) Receptor. (C) Inhibition of S-RBD binding to the ACE2 receptor by neutralizing anti-S-RBD IgG antibodies.
Schematic illustration of postulated mechanism modulating SARS-CoV-2 attachment, fusion and neutralizing activity of anti-S-RBD IgG SARS-CoV-2 antibodies. (A) Structural components SARS-CoV-2: Envelop Protein (E), Membrane glycoprotein (M), Nucleocapside protein (N), RiboNucleic Acid (RNA), Spike protein (S). (B) Binding of Receptor-Binding Domain (RBD) to the Angiotensin-converting enzyme 2 (ACE2) Receptor. (C) Inhibition of S-RBD binding to the ACE2 receptor by neutralizing anti-S-RBD IgG antibodies.
Study findings
Using an indirect chemiluminescence immunoassay, the researchers found that antibodies against S-RBD were lower in individuals who were previously infected as compared to those who were vaccinated, with or without previous infection. Notably, a previous SARS-CoV-2 infection before vaccination was not found to affect the anti-S-RBD IgG levels.
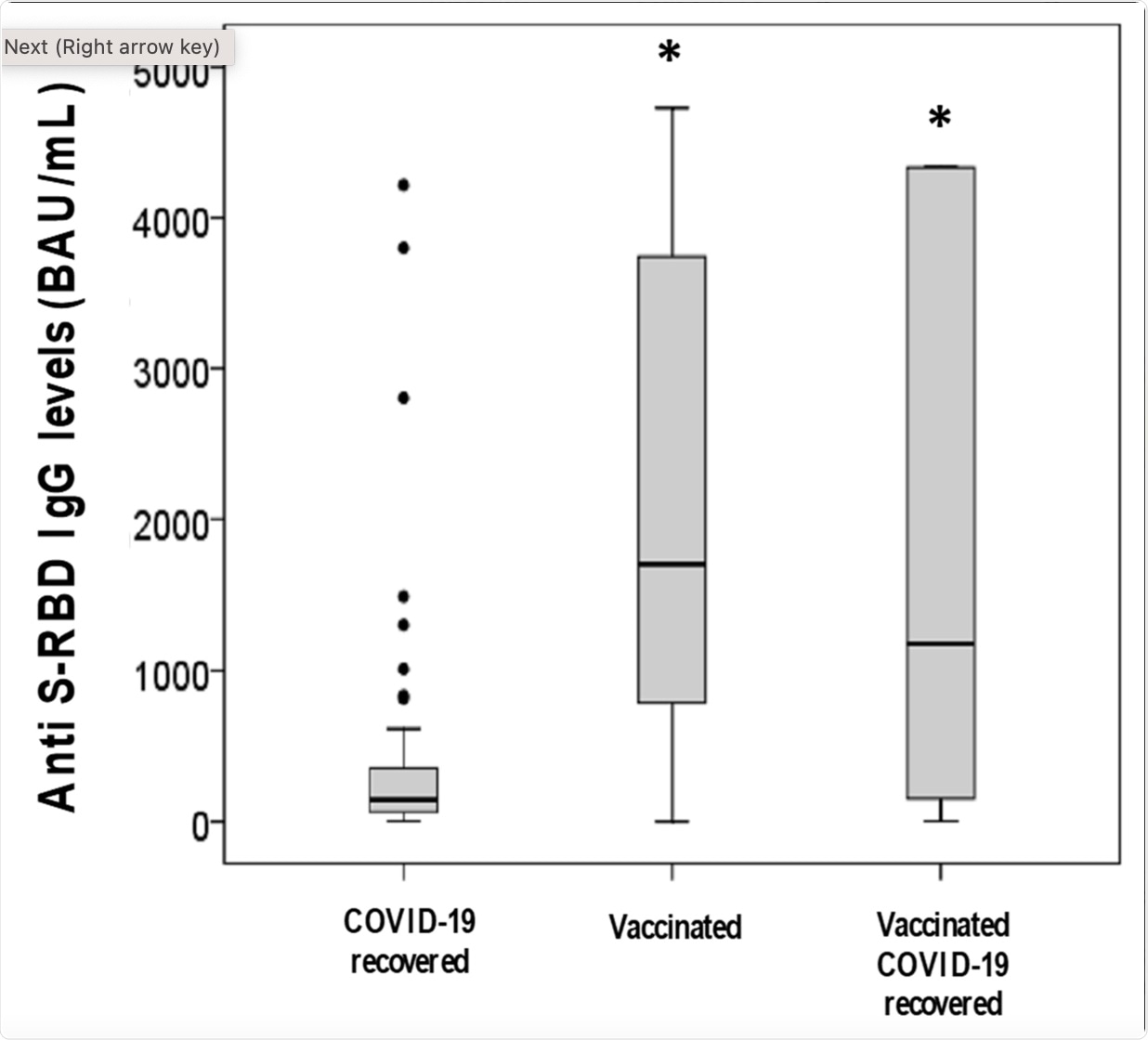 Anti S-RBD IgG levels in the three groups investigated * p < 0.001 vs. “COVID-19 recovered” group, Bonferroni’s correction.
Anti S-RBD IgG levels in the three groups investigated * p < 0.001 vs. “COVID-19 recovered” group, Bonferroni’s correction.
It was also reported that anti-RBD IgG levels were higher in females than males at 2,110 vs. 1,341 binding antibody units (BAU)/mL, respectively.
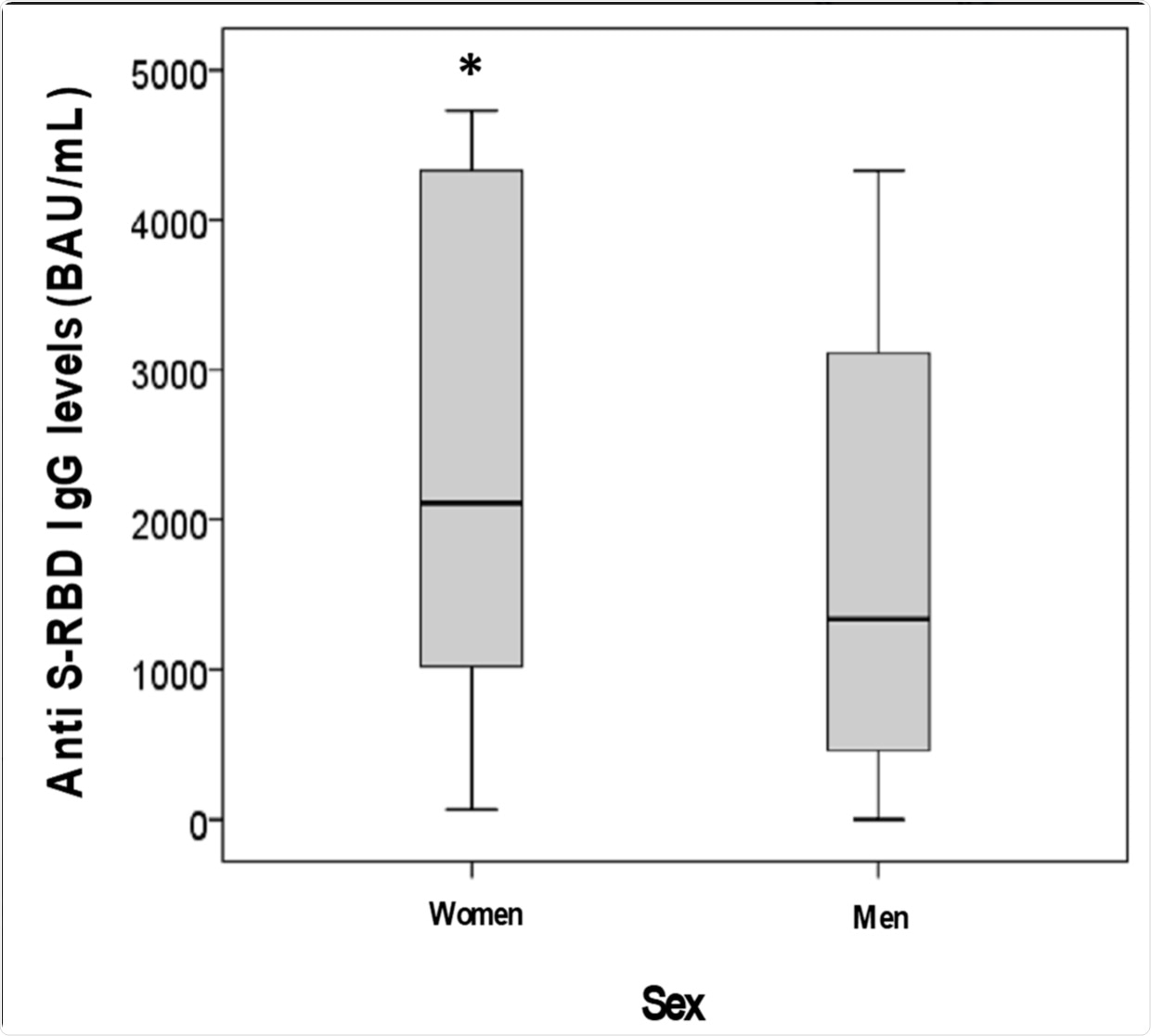 Anti S-RBD IgG levels in subjects sub-grouped according to sex (* p < 0.001 vs. men).
Anti S-RBD IgG levels in subjects sub-grouped according to sex (* p < 0.001 vs. men).
The researchers also reported that antibody levels were higher in individuals who complained of symptoms like local/muscle pain, respiratory stress, and other systemic reactions post-vaccination as compared to those who were asymptomatic after their vaccination. This finding, therefore, affirms that this specific vaccine can successfully elicit robust immune responses. It should also be noted that lower antibody levels were detected in older individuals as compared to younger test subjects.
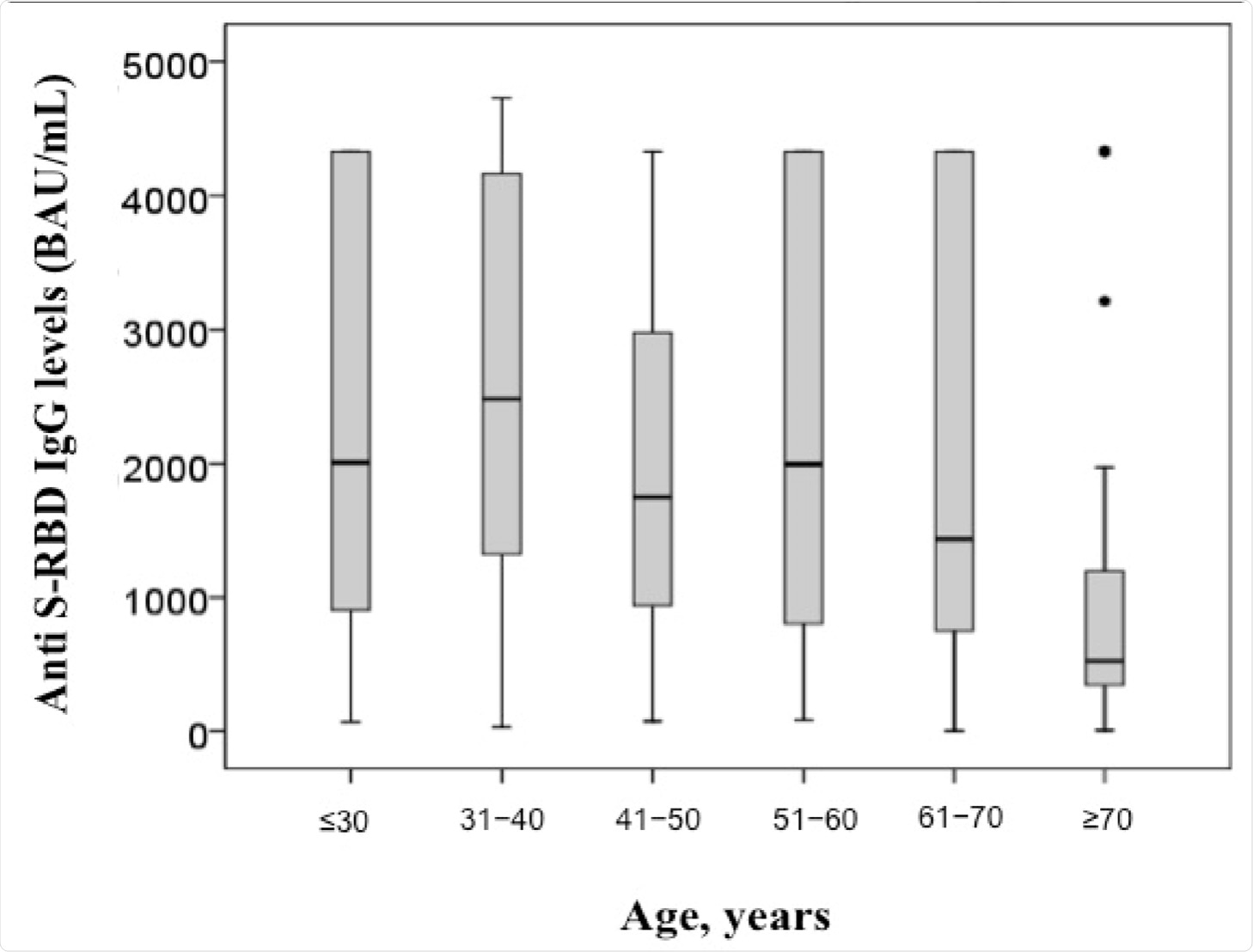 Anti S-RBD IgG levels in subjects sub-grouped by age.
Anti S-RBD IgG levels in subjects sub-grouped by age.
These findings, therefore, support the monitoring of both men and the elderly following their vaccination. To this end, earlier revaccination and/or an increase in the vaccine dose may be warranted to ensure stronger and long-lasting immunity, as well as extended protection against SARS-CoV-2.
The researchers also noted a significant decrease in anti-RBD IgG levels within a short period from a complete two-dose cycle vaccination. This rapid antibody decay rate within a short period after a completed two-dose vaccine cycle was not influenced by age and sex.
Conclusion
“Since the COVID-19 vaccines have been developed recently, other important questions must be addressed, including the durability of protection over a long period after vaccination and the determination of the effect of a booster dose to extend the duration of immunity against SARS-CoV-2 infection.”
To summarize, this large observational study showed that a robust antibody response occurs after the administration of the mRNA BNT162b2 COVID-19 vaccine that was characterized by good antibody production with age- and sex-related differences. Evaluating the humoral response to the vaccine is vital to understand the efficacy of the vaccine and, consequently, to tailor the optimal vaccination strategy.