Despite the rapid global transmission of the SARS-CoV-2 Omicron variant, death rates have not increased, even in highly vaccinated populations. This suggests that immunological mechanisms, other than neutralization, may still provide protection against severe illness.
Study: Preserved Omicron Spike specific antibody binding and Fc-recognition across COVID-19 vaccine platforms. Image Credit: Naeblys / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Leveraging Fc-activity against Omicron
SARS-CoV-2 has acquired a progressive collection of mutations preferentially within the S1 domain of the spike antigen, within or proximal to the receptor-binding domain (RBD). These mutations appear to enhance spike binding to the angiotensin-converting enzyme 2 (ACE2) receptor as it continues to evolve and adapt to its new host.
Both vaccine-induced neutralizing antibodies and monoclonal therapies have gradually lost neutralization potency against developing variants of concern (VOCs). This is largely because many of the strong neutralizing antibodies attach to the RBD in an effort to disrupt or prevent interactions with ACE2. This loss of neutralization, combined with increased ACE2-binding, is responsible for the global increase in transmission events.
However, following infection, both direct and indirect cellular mechanisms contribute to pathogen control and clearance as a second line of defense. T-cells, in particular, may be able to recognize and kill infected cells directly.
Furthermore, binding antibodies that connect with Fc receptors (FcRs), which are located on immune cells, can boost the innate immune system's antiviral function. This causes quick opsonophagocytic clearance, infected cell cytotoxicity, or pro/anti-inflammatory mediators, among other things, all of which have been related to protection against viruses such as influenza, Ebola, human immunodeficiency virus (HIV), and, most recently, SARS-CoV-2.
About the study
In the present study, researchers demonstrate that while antibody isotype binding to the Omicron RBD decreased across vaccine platforms, substantial Fc-activity to the Omicron spike remained. This likely contributes to the quick control and clearance of viral infection and reduced illness severity.
To compare antibody responses evoked by the various vaccines, samples were taken at peak immunogenicity timepoints from people who had received the complete dose regimen indicated by the manufacturer. Luminex multiplexing was used to look at antigen-specific antibody subclasses and isotypes, as well as FcγR binding.
Study findings
The researchers found a more dramatic loss of Omicron-RBD as compared to Omicron-spike isotype/subclass. FcR binding was also seen across vaccination platforms, which is likely due to the preferential integration of changes in the SARS-CoV-2 spike's S1 domain.
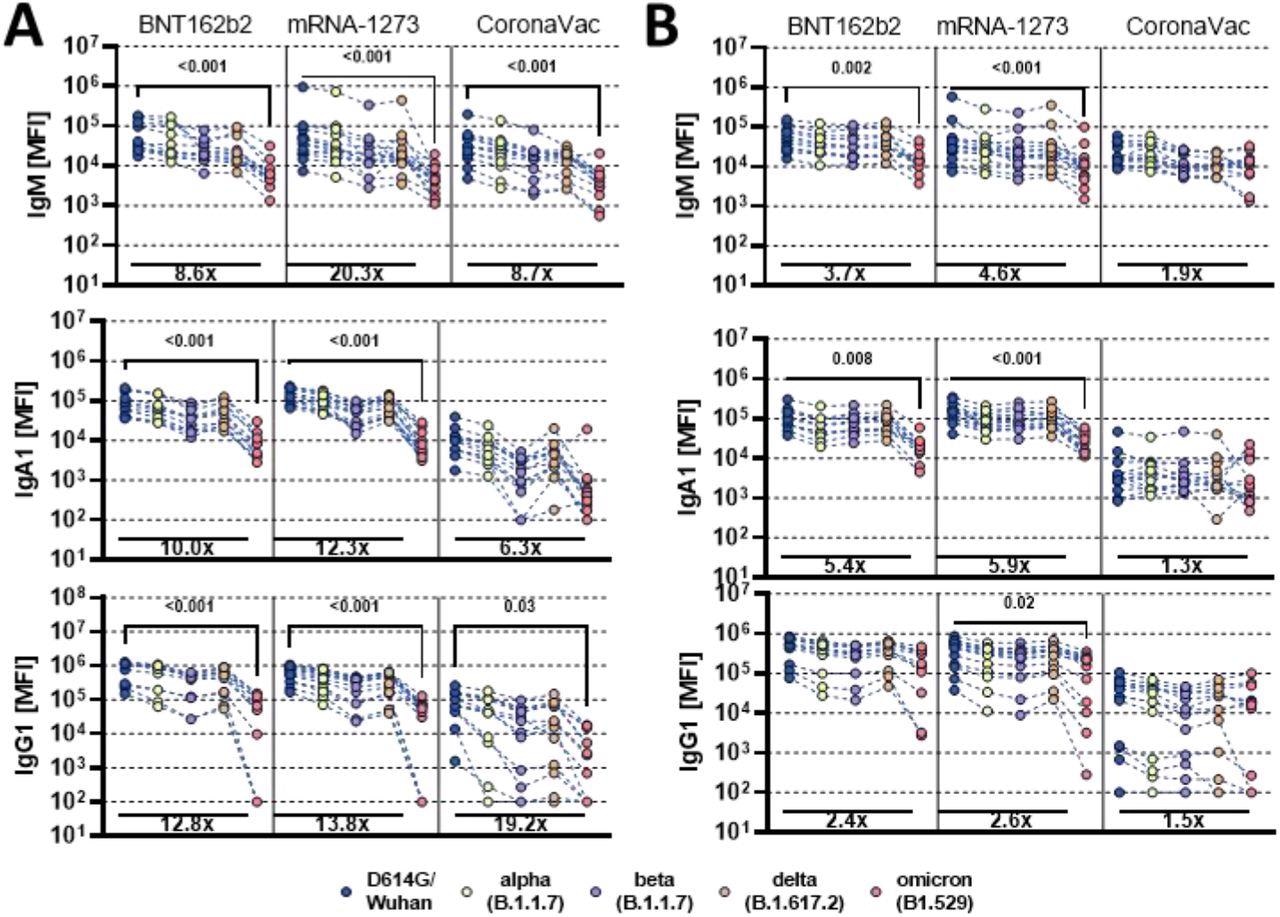
Vaccine-induced antibody binding to different SARS-CoV-2 variants of concern. Individuals either received the full dose regimen of the BNT162b2(n = 11), mRNA-1273(n=14), or the aluminum adjuvanted inactivated particle vaccine CoronaVac (n=13). Samples were taken at peak immunogenicity 2 weeks after the last dose. IgM, IgA1 and IgG1 binding titers to D614G (WT), Alpha (B1.117), Beta (B1.351), Delta (B.1.617.2), and Omicron (B1.529) variants of concern receptor-binding domain (A) or full Spike (B) were measured by Luminex. Background corrected data is shown and negative values were set to 100 for graphing purposes. A Kruskal-Wallis test with a Benjamini-Hochberg post-test correcting for multiple comparisons was used to test for statistical differences between wildtype variant and omicron titer. P-values for significant different features are shown above and fold change reduction of omicron titer compared to wildtype below each dataset.
Antibodies that mediate Fc-activity, on the other hand, can likely bind throughout the full antigenic surface. This is unlike neutralizing antibodies, which must target a restricted number of areas on the spike protein that are involved in attachment, positioning of the RBD, or fusion.
The development of immune complexes and the organization of antibodies with Fc-domains that are accessible to local immune cells are required for Fc activity. Omicron-immunoglobulin G (IgG) binding to the spike antigen was found to be persistent across messenger ribonucleic acid (mRNA) and inactivated vaccination platforms.
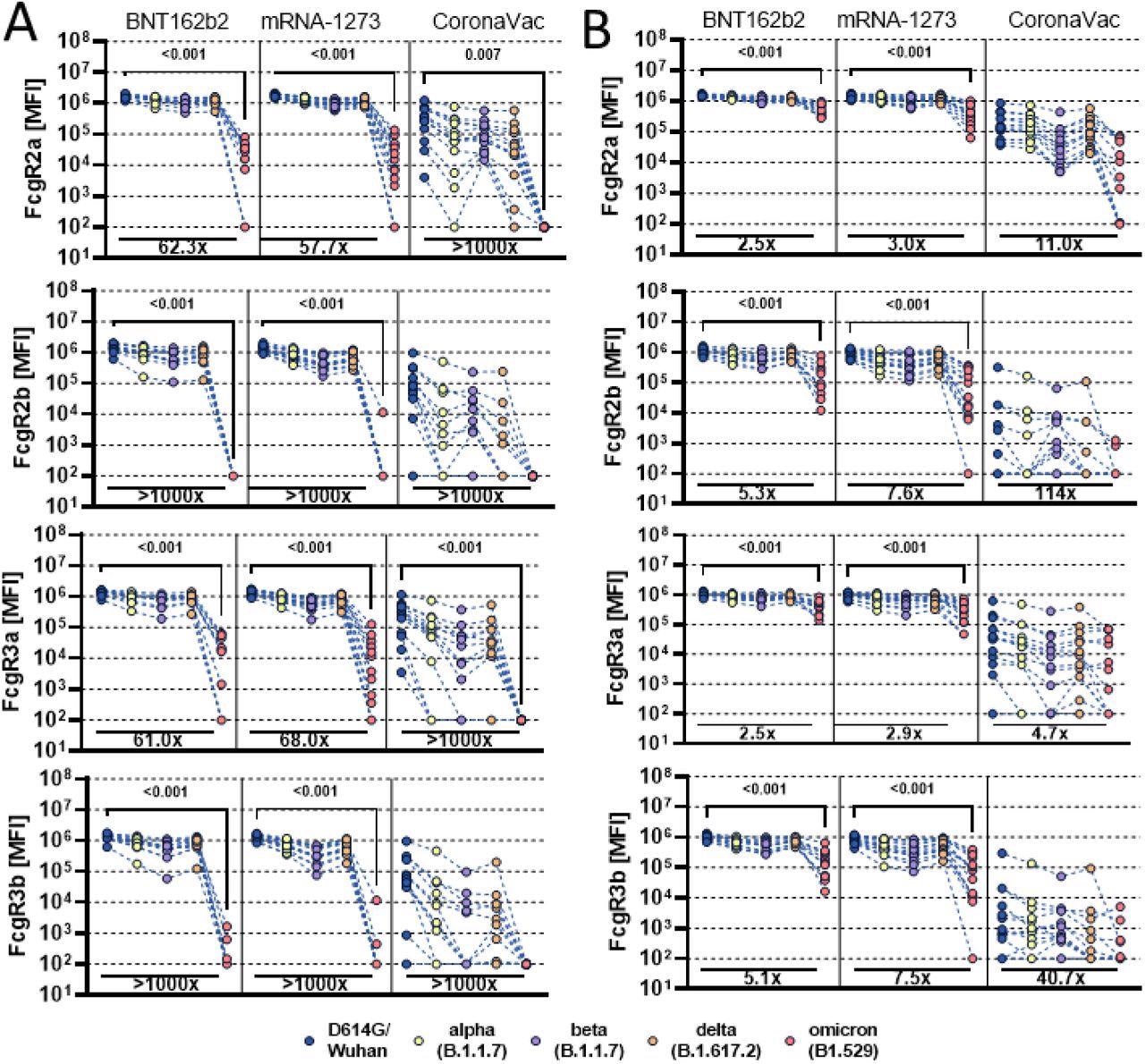
Vaccine-induced Fcγ-receptor binding antibody profiles across SARS-CoV-2 variants of concern. Individuals either received the full dose regimen of the BNT162b2(n = 11), mRNA-1273(n=14), or the aluminum adjuvanted inactivated particle vaccine CoronaVac (n=13). Samples were profiled at peak immunogenicity 2 weeks after the last dose. Binding to FcγR2a, FcγR2b, FcγR3a and FcγR3b of D614G (WT), Alpha (B1.117), Beta (B1.351), Delta (B.1.617.2), and Omicron (B1.529) variant of concern receptor-binding domain (A) or full Spike (B) specific antibodies were determined by Luminex. Background corrected data is shown and negative values were set to 100 for graphing purposes. A Kruskal-Wallis test with a Benjamini-Hochberg post-test correcting for multiple comparisons was used to test for statistical differences between wildtype variant and omicron titer. P-values for significant different features are shown above and fold change reduction of omicron titer compared to wildtype below each dataset.
This suggests that vaccine-induced antibodies may continue to opsonize the virus and virally infected cells, even after infection with Omicron. While neutralizing antibodies are likely to be important in preventing transmission, non-neutralizing antibodies that take advantage of Fc-biology may help to maintain disease attenuation.
While the three BNT162b2, mRNA-1273, and CoronaVac vaccines retained stronger binding to the Omicron spike specific FcgR2a, there was a selective loss of FcgR2b and FcgR3b across all platforms. In humans, FcgR2b is the only low-affinity inhibitory receptor and is thought to contribute to reduced inflammatory activity.
Similarly, FcgR3b is expressed exclusively on neutrophils and is thought to be important for rapid opsonophagocytic clearance of opsonized virus particles. While ongoing binding to FcR2a and FcR3a may result in continued particle clearance and infected cell killing, the loss of FcR2b and FcR3b may lead to increased inflammatory response, which may translate to symptoms and may still result in death.
In conclusion, identifying the immunological pathways that lead to illness attenuation in the absence of neutralization could provide crucial information for developing efficient pan VOC vaccines and increasing campaigns to combat the global COVID-19 pandemic.
“While neutralizing antibodies are likely to be key to blocking transmission, non-neutralizing antibodies able to 157 leverage Fc-biology may contribute to persistent disease attenuation.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Bartsch, Y., Tong, X., Kang, J., et al. (2021). Preserved Omicron Spike specific antibody binding and Fc-recognition across COVID-19 vaccine platforms. doi:10.1101/2021.12.24.21268378. https://www.medrxiv.org/content/10.1101/2021.12.24.21268378v1.
- Peer reviewed and published scientific report.
Bartsch, Yannic C., Xin Tong, Jaewon Kang, María José Avendaño, Eileen F. Serrano, Tamara García-Salum, Catalina Pardo-Roa, et al. 2022. “Omicron Variant Spike-Specific Antibody Binding and Fc Activity Is Preserved in Recipients of MRNA or Inactivated COVID-19 Vaccines.” Science Translational Medicine, March. https://doi.org/10.1126/scitranslmed.abn9243. https://www.science.org/doi/10.1126/scitranslmed.abn9243.