The induction of hybrid immunity after SARS-CoV-2 exposure has hampered the establishment of serum-neutralizing antibodies (nAbs) as a mechanistic correlate of protection (CoP) against COVID-19. Thus, even after performing multiple studies on COVID-19 vaccinated and convalescent cohorts, researchers have not been able to define a protective nAb threshold against COVID-19.
MAbs are a crucial part of the arsenal against SARS-CoV-2. However, serum-mAb titers conferring protection against symptomatic COVID-19 are yet unknown.
About the study
The researchers had hitherto engineered a broadly neutralizing antibody (bnAb), ADG2, targeted towards the receptor-binding domain (RBD) region of SARS-CoV-2 for which they derived the precursor from a SARS-CoV survivor of the 2003 outbreak. In vitro-engineered mAb, ADG2 demonstrated high potency against all sarbecoviruses targeting host angiotensin-converting enzyme 2 (ACE2) receptors.
In the present study, they made a two–amino acid alteration in the fragment crystallizable (Fc) region of ADG2 and named the resultant mAb, adintrevimab. This new mAb had enhanced binding affinity to the neonatal Fc receptor (FcRn) at pH=6 and a prolonged serum half-life in vivo. In addition, its binding affinity to cynomolgus macaque and human FcRn was five and six-fold higher than ADG2, respectively.
The team also assessed the pharmacokinetics (PK) of adintrevimab in SARS-CoV-2-naïve cynomolgus macaques. To this end, they administered a 10 mg/kg intramuscular (IM) injection or intravenous (IV) infusion of adintrevimab and followed up for up to 98 days. It gave the researchers a preliminary idea of the neutralization profile, biophysical properties, and half-life of adintrevimab in non-human primates (NHPs).
Based on its performance in the NHP animal model, the team performed a phase II/III study in SARS-CoV-2–naïve adult individuals (humans). It helped them assess the safety and PK of a 300mg IM dose of adintrevimab against symptomatic COVID-19 within the preexposure prophylaxis (PrEP) cohort. They also investigated the association between serum-neutralizing titers due to adintrevimab and its protective efficacy.
In this human trial, the primary analysis cohort comprised seronegative individuals recruited between April 2021 and January 2022, the transition period when Omicron BA.1/BA1.1 became predominant. The primary efficacy endpoint was the occurrence of symptomatic COVID-19, confirmed by reverse transcription polymerase chain reaction (RT-PCR) after adintrevimab or placebo administration up to day 90.
Per whole-genome sequencing (WGS) results,~98% of infections in the pre-Omicron cohort were due to Delta, whereas ~90% of those in the Omicron cohort were due to Omicron BA.1 or BA1.1 subvariants.
Results
In the pre-Omicron cohort, i.e., the participants enrolled on or before 30 November 2021, adintrevimab administration reduced the risk of symptomatic COVID-19 by 71% compared to placebo for up to three months. However, due to the relatively lower potency of adintrevimab against Omicron, its efficacy diminished more rapidly in the Omicron PrEP cohort. Thus, the researchers observed a relative reduction in the risk of symptomatic COVID-19 from 60% to 41% from day 28 to day 60. At day 90, it went down to 37% vs. placebo at day 90.
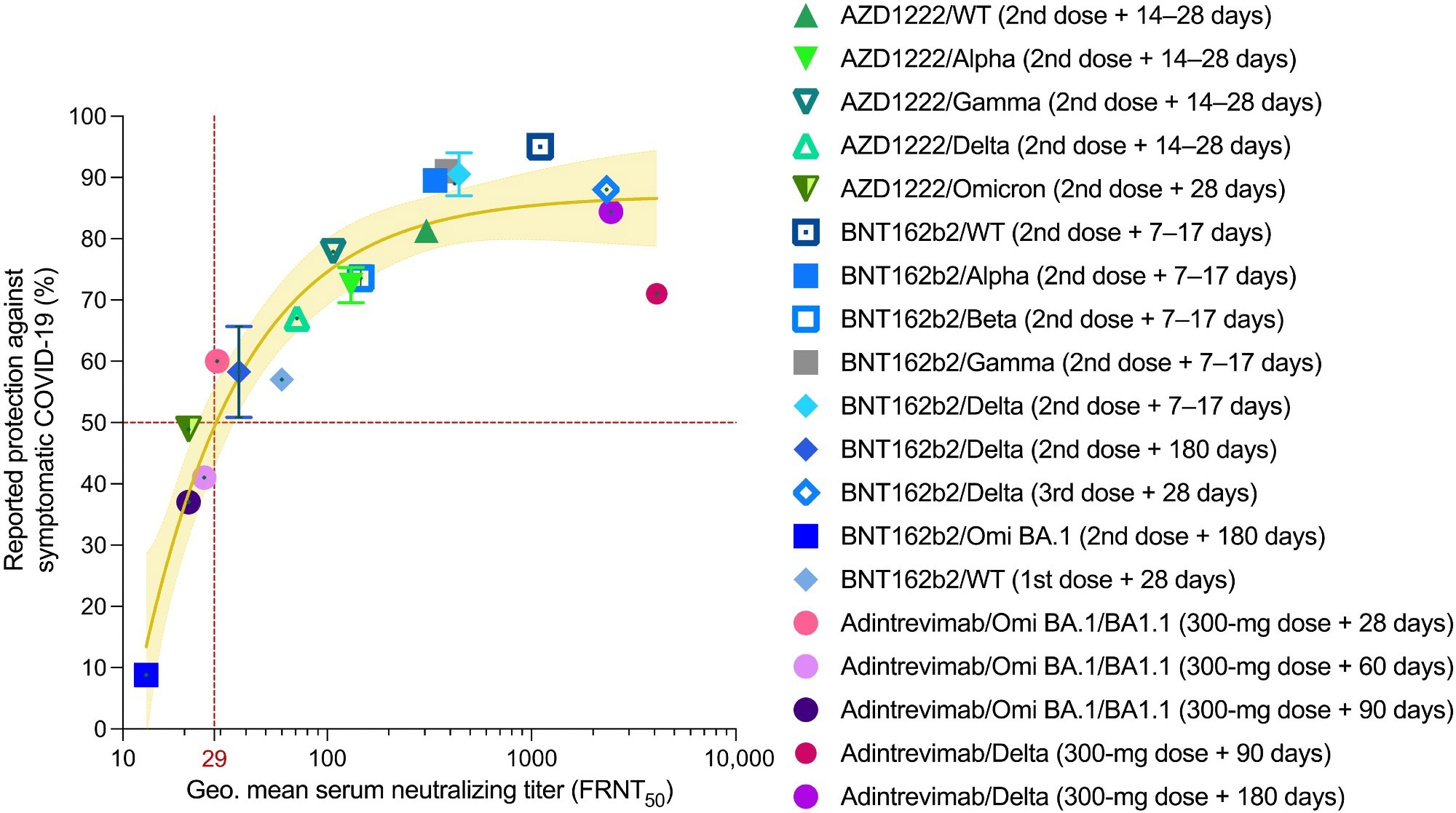
Serum nAb titers are predictive of protection against symptomatic COVID-19. Reported median vaccine-induced serum neutralizing titers and normalized monoclonal antibody neutralizing titers (adintrevimab), measured in an authentic virus neutralization assay are plotted against reported efficacy in phase 2/3 clinical trials or real-word vaccine effectiveness studies at the time points indicated in the legend. The brown solid line indicates the best fit of the nonlinear regression, and the yellow shading indicates 95% confidence intervals. Data points and error bars represent means ± SD. The neutralizing titer associated with 50% protection against symptomatic COVID-19 is indicated by a vertical dotted red line. Omi, Omicron; Geo., geometric.
Normalizing the study population to PK model–derived average serum concentrations of adintrevimab helped the researchers determine virus neutralization data for Omicron BA.1/BA1.1 and Delta. They extrapolated serum nAb titers against these SARS-CoV-2 variants, where serum-neutralization titer equaled adintrevimab serum concentration or variant-specific half-maximal inhibitory concentration (IC50).
On days 90 and 180, the normalized Delta serum neutralizing titers correlated with 71% and 84.4% risk reductions against symptomatic Delta infection. Likewise, on days 28, 60, and 90, the average serum concentrations of adintrevimab translated to anti-Omicron BA.1 serum neutralizing titers of 1:29, 1:25, and 1:21, respectively. These neutralizing titers corresponded to 60%, 41%, and 37% relative risk reductions against symptomatic Omicron BA.1/BA1.1 infection in humans. Together, these results demonstrated that adintrevimab protected symptomatic COVID-19 even at serum nAb titers as low as ~1:30.
Conclusions
The study gathered enough evidence that nAbs are mechanistic CoP and conferred a high level of protection even at low serum nAb titers, even in the absence of T cell and memory B cell responses. However, the researchers could not define the absolute protective serum neutralization threshold because breakthrough infections occurred in patients at time points associated with very high serum nAb titers.
The authors acknowledged that Fc-dependent effector functions of adintrevimab might be at play in conferring protection, especially in the context of non-RBD antibodies, e.g., nAbs targeting the spike (S) subunit 2. Combined with PK data from several human studies, the study data suggested that adintrevimab could confer at least 50% protection against symptomatic disease by susceptible SARS-CoV-2 variants for up to three years. Together, these results support the use of half-life extended mAbs for SARS-CoV-2 prophylaxis, especially in populations at a higher risk.
The high efficacy of adintrevimab against symptomatic disease by highly lethal Delta and immune-evading Omicron suggests that broad-acting, highly potent, and half-life extended mAbs offer more durable protection than currently available COVID-19 vaccines. Amid the rapid evolution of SARS-CoV-2 might make this mAb ineffective like Omicron did to the previously approved SARS-CoV-2 mAb therapeutics, the future challenge would be the development of next-generation mAb therapeutics.
Journal reference:
- Antibody-mediated protection against symptomatic COVID-19 can be achieved at low serum neutralizing titers, Pete Schmidt, Kristin Narayan, Yong Li, Chengzi I. Kaku, Michael E. Brown, Elizabeth Champney, James C. Geoghegan, Maximiliano Vásquez, Eric M. Krauland, Thomas Yockachonis, Shuangyi Bai, Bronwyn M. Gunn, Anthony Cammarata, Christopher M. Rubino, Paul Ambrose, Laura M. Walker, Science Translational Medicine 2023, DOI: 10.1126/scitranslmed.adg2783, https://www.science.org/doi/10.1126/scitranslmed.adg2783