Researchers reveal that continuous monitoring with the STAT-ON™ wearable could transform Parkinson’s management, saving millions in care costs while enhancing patients’ quality of life.
 Study: Improving Parkinson’s disease management through wearable technology: A cost-benefit perspective. Image credit: Chinnapong/Shutterstock.com
Study: Improving Parkinson’s disease management through wearable technology: A cost-benefit perspective. Image credit: Chinnapong/Shutterstock.com
STAT-ON™, a wearable medical device, may reduce clinical costs related to Parkinson’s disease management by continuously monitoring symptoms and optimizing treatment adjustments, as a new study published in PLOS ONE reported.
Background
Parkinson’s disease (PD) is an age-related neurodegenerative disease, affecting approximately 7 to 10 million people globally. With increasing life expectancy and related rise in aging populations, especially in Western countries, the prevalence of PD is expected to reach 17 million in 2040 and 25 million in 2050.
Such an exponential rise in prevalence, together with a high increase in medical costs, may significantly burden public healthcare systems. According to the Parkinson’s Europe Association, the social costs of PD are approximately €11.600 per patient per year.
The diagnosis of PD is a complex process, and no curable treatment is currently available. Existing evidence indicates that about 40% of PD cases are wrongly diagnosed in current clinical practice, which is often frustrating for both patients and physicians.
In this context, many neurologists suggest that wearable medical devices can be helpful in continuously monitoring symptoms and acquiring relevant and reliable information from patients in home environments. However, the cost-effectiveness of introducing wearable devices in clinical practice remains largely unknown.
In the current study, researchers explored the modeled economic impact and potential clinical use of a wearable medical device, STAT-ON™, to reduce overall healthcare costs and improve quality of life in patients with PD.
STAT-ON™
STAT-ON™ is an artificial intelligence (AI)-powered waist-worn medical device that monitors PD-related motor symptoms during daily activities. It also provides information about patient’s medication intake.
PD symptoms are often underestimated in clinical practice due to subjective assessments and limited consultation time. Clinical evaluation is also challenging, as symptoms typically occur every 6 to 9 months and last only about 20 minutes per visit. This lack of diagnosis subsequently leads to delayed treatment adjustments and worsening of clinical outcomes and patients’ quality of life.
STAT-ON™ is an extensively validated device that can accurately detect key motor symptoms related to PD, supporting physicians in treatment decision-making and optimization. The device has shown comparable efficacy in hospital settings with other conventional tools, highlighting its clinical usefulness in assisting hospital neurologists.
The patient’s data is securely stored in the device with proper privacy, and data retrieval is managed through a healthcare professional’s smartphone app. After retrieval and report generation, the data is deleted for device reuse with other patients.
One major advantage of STAT-ON™ over wrist-worn devices is that its placement on the waist enables more precise monitoring of axial symptoms. On the other hand, wrist-worn devices cannot accurately monitor motor complications affecting the trunk, lower limbs, and neck.
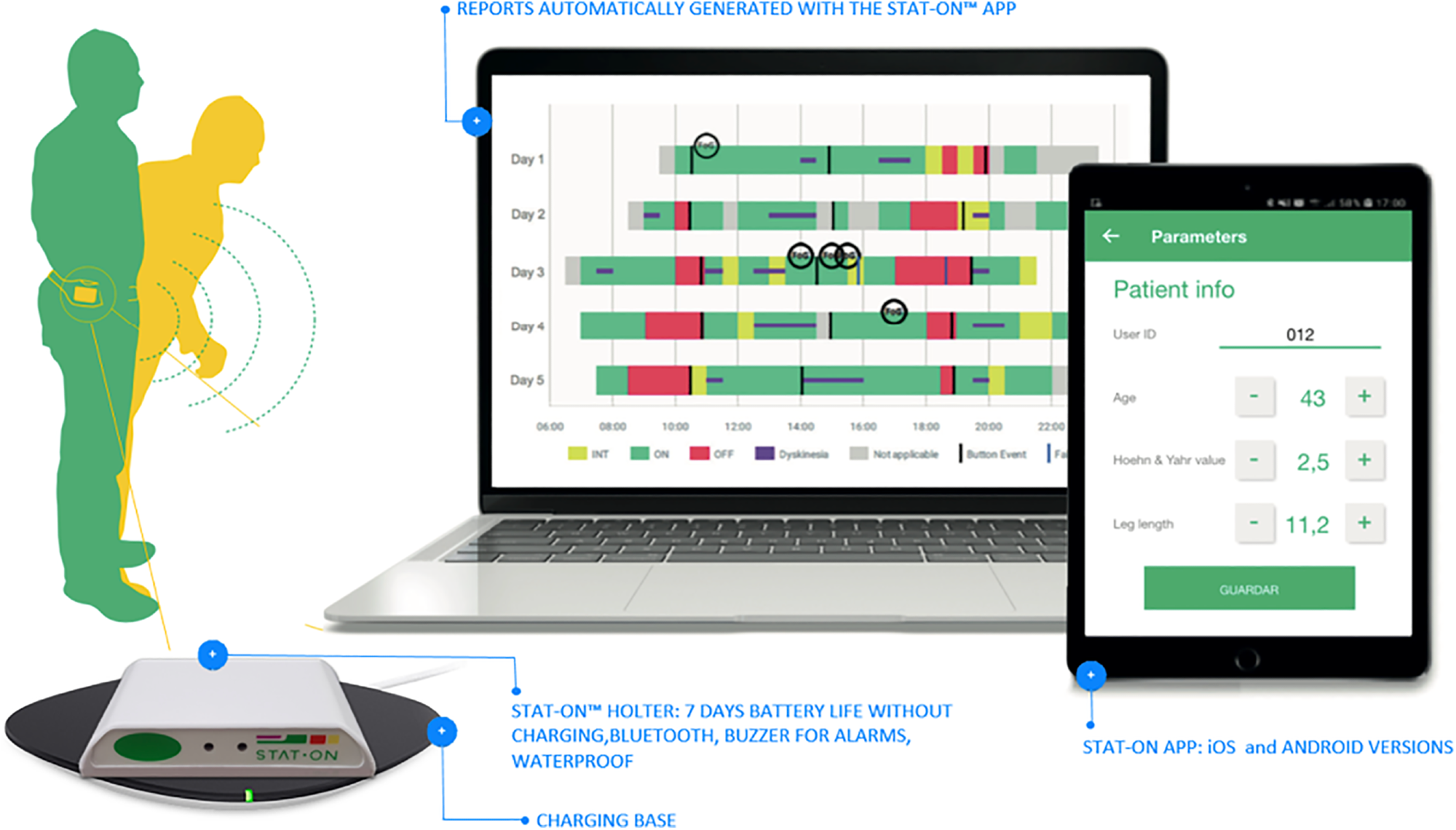 STAT-ON™ general view: The accompanying App enables configuring it at the beginning and generating the final report after the observation.
STAT-ON™ general view: The accompanying App enables configuring it at the beginning and generating the final report after the observation.
Cost-benefit analysis of STAT-ON™
The study used previously published and validated data to conduct a cost-benefit analysis for introducing STAT-ON™ in European healthcare systems for PD management.
The analysis revealed that continuous monitoring and accurate assessment of PD symptoms using STAT-ON™ helps optimize treatment regimens, reducing hospitalizations and institutional care costs.
Studies in different European countries reported considerable healthcare cost reductions through STAT-ON™ mediated detection of moderate and advanced symptoms in patients with PD. Specifically, the study estimated potential cost reductions of up to €137.8 million in Germany and €19 million in Sweden when STAT-ON™ is used to detect advanced PD symptoms.
These benefits support the inclusion of STAT-ON™ in clinical practice. Although this inclusion may increase medication expenses due to the prescription of highly expensive medicines for advanced disease stages, the improvement in patients’ quality of life and reduction in direct healthcare expenses will help offset the costs of medications.
Utility of STAT-ON™
STAT-ON™ was developed to reduce healthcare costs and improve patients' quality of life. This device can also serve as a telemedicine tool for remote monitoring of PD patients. Telemedicine studies involving STAT-ON™ have reported significant improvement in daily-life activities, physical activity, depression, apathy, movement difficulties, balance, and frailty in PD patients.
Besides clinical benefits, STAT-ON™ can support fundamental research by providing data on PD patients’ motor symptoms and daily activities. By ensuring personalized monitoring, the device can also reduce participant drop-out and improve adherence, strengthening the quality of clinical trials.
Regarding treatment optimization, STAT-ON™ can assist physicians in identifying patients suitable for second-line therapies, such as deep brain stimulation, continuous dopaminergic infusions, and other advanced treatments recommended for patients who do not respond to first-line medications.
Overall, the study's model-based cost-benefit analysis supports including STAT-ON™ in clinical practice for PD management. However, the analysis relies on economic data from various European healthcare systems, which differ in structure, reimbursement policies, and medical care costs, potentially limiting the generalizability of the findings.
Furthermore, the study addressed direct medical costs related to PD. Still, it did not thoroughly consider indirect costs such as caregiver burden and lost productivity, which may affect the observed cost-effectiveness of STAT-ON™.
Previous validation studies determined the efficacy of STAT-ON™ in detecting PD symptoms. Further large-scale clinical trials are required to confirm the effectiveness across different patient populations.
Download your PDF copy now!