The clinical phenotype of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is remarkable for its wide range of severity among individual patients. Genetic variations are known to mediate part of these differences.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study, published as a preprint on the bioRxiv* server, uses a panel of iPSCs from over 500 individuals. The researchers preferred undifferentiated iPSCs to reduce the time required to differentiate them, especially since infection is not always reliable.
Establishing SARS-CoV-2 infection in iPSCs
Human iPSCs are not infected by SARS-CoV-2 since they express the viral entry receptor, the angiotensin-converting enzyme 2 (ACE2) at low levels. These cells do have high levels of the coronavirus receptor CD147 and of the serine protease TMPRSS2.
In order to render them susceptible to SARS-CoV-2 infection, the iPSCs were engineered with the relevant genes, inserted by adenovirus vectors (Ad), with almost 100% efficiency. These genes encoded human ACE2 and TMPRSS2 at over-expressing levels, leading to massive infection by the virus. TMPRSS2 overexpression alone was inadequate to produce this effect.
Two days post-infection of the ACE2-positive iPSCs, cell fusion was observed, with cell death after four days, in many cases. Under transmission electron microscopy (TEM), the infected cells showed characteristic changes, including zippered endoplasmic reticulum (ER), double-membrane spherules (DMS), and viral particles near the cell membrane.
Viral particles were seen within the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), along with double-membrane vesicles (DMVs. The latter is central to the synthesis of viral RNA and is absent from uninfected cells.
Thus, TEM is a useful tool to examine the lifecycle of this virus.
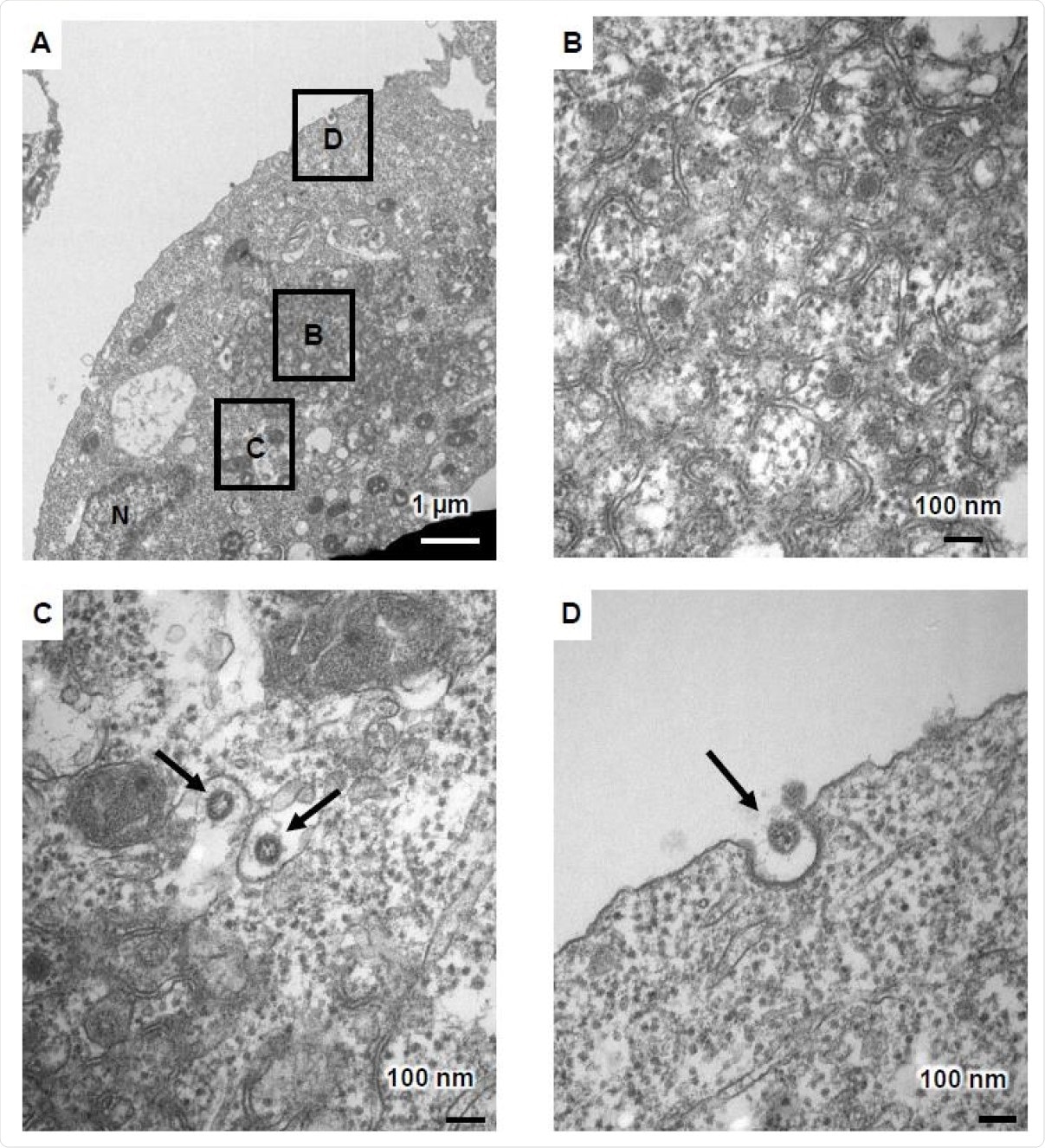
TEM images of infected ACE2-iPS cells (A) TEM images of infected ACE2-iPS cells. Zippered endoplasmic reticulum, double-membrane spherule (DMS) (B), and virus particles in the ERGIC (black arrows) (C) and near the cell membrane (black arrows) (D) were observed.
Undifferentiated state persists following infection
Gene and protein expression was assessed on day 3 post-infection in both infected and uninfected cells. This showed that infected cells had high levels of the viral genome and of ACE2, but not of undifferentiated cell markers or of innate immunity markers. The viral nucleocapsid (N) protein was also strongly expressed in the infected cells on day 2 post-infection.
RNA-seq analysis of these cells showed that in about 7% of all genes, there was a four-fold or more change in the level of expression, and none of these encoded markers of undifferentiation or innate immune markers.
Endoderm markers, except for the CER1 gene and for CD147, NRP1, and TMPRSS2 genes, known to be SARS-CoV-2-related markers, also remained unchanged. Mesoderm and ectoderm markers were also unchanged.
These results suggest that human iPS cells maintain an undifferentiated state even when SARS-CoV-2 replicates in large numbers.”
Candidate drug evaluation
In comparison to Vero cells, iPSCs proved to show stronger drug effects on SARS-CoV-2 infection, except for interferon-beta. Of the eight drugs tested, remdesivir showed maximum antiviral activity, and ivermectin showed high cytotoxicity.
Chloroquine and Favipiravir failed to suppress viral replication. The RNA-dependent RNA Polymerase (RdRp) inhibitors (Remdesivir and EIDD-2801) and TMPRSS2 inhibitors (Camostat and Nafamostat) were proved to have antiviral activity in these cells, indicating their value in drug evaluation
Individual differences
The researchers then infected iPSCs from eight different donors to explore differences in infection due to purely individual factors. The virus was replicated with varying efficiency among the eight donor cell lines, despite the comparable expression of ACE2 in all of them.
The virus showed greater replication capacity in male-origin cells than females, indicating the potential for sex-mediated differences in susceptibility to be replicated in these cells. Both the androgen receptor and its target TMPRSS2 gene, of which the latter is expressed at slightly higher levels in male iPSCs, may be implicated in this difference.
What are the implications?
The viral lifecycle can be studied in these human iPSCs if the ACE2 receptor is overexpressed. The study showed the operation of sex-specific differences in the efficiency of infection and of viral replication. The inhibitory effects of drugs were also observable in this cell model.
These results suggest that by using our iPS cell panel, it will be possible to investigate the effects of race and blood type as well as gender on SARS-CoV-2 infection.”
Furthermore, the identification of genetic mutations occurring at high frequency in cells that sustain infection is made possible by the use of iPSCs from a panel with documented genomic information.
While the infection efficiency varies among different donors, the fact that none of these donors had COVID-19 limits the inference that they had varying susceptibility to the disease. The study is being taken forward by using iPSCs from patients with mild and severe COVID-19, allowing findings in infected cells to be compared with the clinical presentation of the patient.
While mutations in certain innate immune genes may be associated with symptom severity, further research is required to understand how these genes function during COVID-19. This can be conveniently done in iPSCs, which allow the easy introduction of single nucleotide mutations.
It is noteworthy that the role of ACE2 overexpression has not been ruled out in the individual differences in SARS-CoV-2 infection within iPSCs from different donors. If so, another system employing ACE2-expressing somatic cells must be used to first assess the effect of the expression and mutation of ACE2 and other related genes.
Causes of individual differences, other than genetic, are not addressed by this system, including those due to advancing age and associated reductions in CD8 T cell cytotoxicity. Studies on blood specimens may help establish the cause of such variations in susceptibility and viral replication. The ACE2-positive iPSCs remain a useful platform to examine and decide on the cause of individual variations, identify high-risk groups and evaluate new drugs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sano, E. et al. (2021). Modeling SARS-CoV-2 infection and its individual differences with ACE2-expressing human iPS cells. bioRxiv preprint. doi: https://doi.org/10.1101/2021.02.22.432218, https://www.biorxiv.org/content/10.1101/2021.02.22.432218v1
- Peer reviewed and published scientific report.
Sano, Emi, Sayaka Deguchi, Ayaka Sakamoto, Natsumi Mimura, Ai Hirabayashi, Yukiko Muramoto, Takeshi Noda, Takuya Yamamoto, and Kazuo Takayama. 2021. “Modeling SARS-CoV-2 Infection and Its Individual Differences with ACE2-Expressing Human IPS Cells.” IScience 24 (5): 102428. https://doi.org/10.1016/j.isci.2021.102428. https://www.cell.com/iscience/fulltext/S2589-0042(21)00396-5.