Aging is associated with cognitive decline and dementia, with poor cognitive function increasing the risk of stroke and Alzheimer’s disease. A reduced cognitive reserve is associated with decreased premorbid cognitive function that exacerbates cognitive impairment.
To date, the exact mechanism that links cognitive decline to dementia is not fully understood. Thus, elucidating the pathogenesis of cognitive decline could support the development of effective strategies to prevent and treat dementia and stroke.
Many studies have recently described the importance of investigating how cognition and structural brain phenotypes are influenced by plasma proteins. However, most of these studies have only assessed a small set of proteins.
About the study
The current study evaluates the association between plasma proteins and cognitive function using data from the Prospective Urban and Rural Epidemiology (PURE) biomarker sub-study. The study cohort included participants with Latin, European, or Persian ancestry. All non-European participants were excluded from the cohort to align the PURE genetic data with external genetic datasets.
Participants who developed stroke, heart failure, type II diabetes, myocardial infarction, or death from any cause were selected for the current study based on random sampling. The PURE biomarker study also invited European participants 39 years and older to enroll in the PURE-MIND study, the latter of which consisted of participants who do not have a history of dementia, stroke, or other neurological disease.
Samples obtained from PURE study participants were used to detect protein quantitative trait loci (pQTLs) for Mendelian randomization analyses, whereas PURE-MIND data were used for observational association analyses. The replication of the observational findings was sought in the Generation Scotland (GS) cohort.
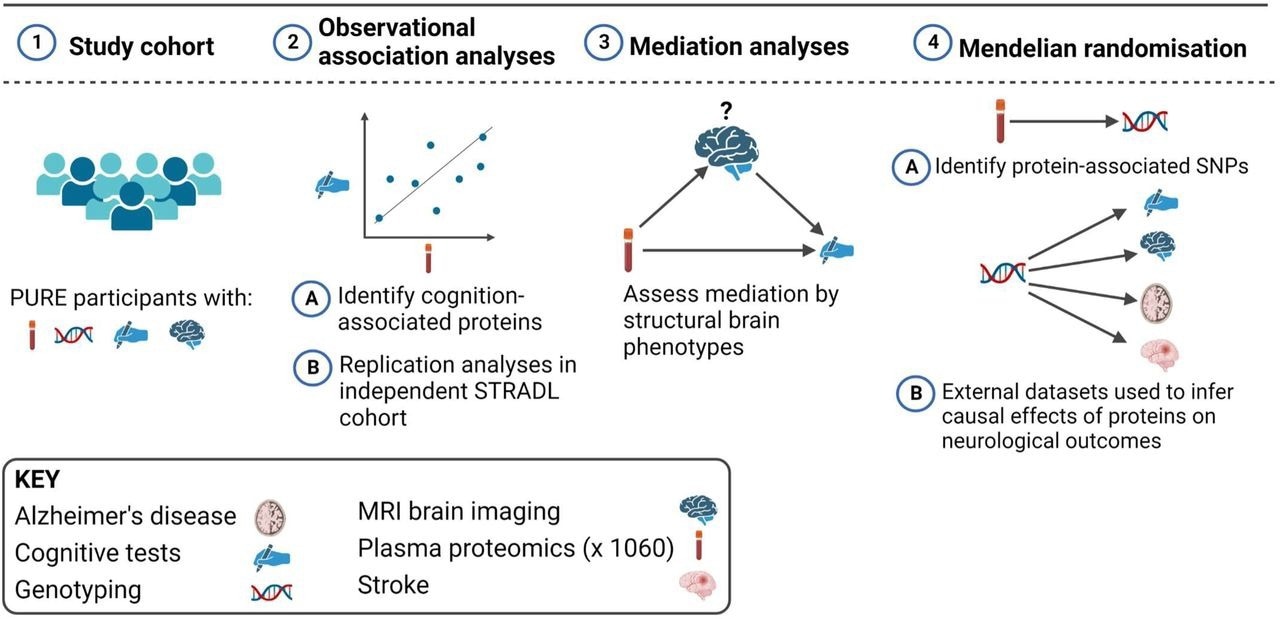 Overview of the study design. This study involved European (N = 3514), Latin (N = 4309), and Persian (N = 1332) PURE participants for whom genetic and plasma proteomic data were available. Observational analyses to detect plasma biomarkers of cognitive function were performed in the subset of these participants who were enrolled in the PURE-MIND sub-study (N = 1198), for whom plasma protein (N = 1060 proteins) and MRI measurements were available. Mediation analyses were performed to assess whether any observed associations between protein levels and cognitive function were mediated by structural brain phenotypes ascertained by MRI. Finally, two-sample Mendelian randomisation analyses were performed to assess potentially causal effects of genetically-predicted cognition-associated protein levels on genetically-predicted neurological outcomes. For these analyses, genetic instrumental variables for protein levels were identified in the European, Latin, and Persian PURE participants, and associations with neurological outcomes were assessed using external (non-PURE) datasets. Created with BioRender.com.
Overview of the study design. This study involved European (N = 3514), Latin (N = 4309), and Persian (N = 1332) PURE participants for whom genetic and plasma proteomic data were available. Observational analyses to detect plasma biomarkers of cognitive function were performed in the subset of these participants who were enrolled in the PURE-MIND sub-study (N = 1198), for whom plasma protein (N = 1060 proteins) and MRI measurements were available. Mediation analyses were performed to assess whether any observed associations between protein levels and cognitive function were mediated by structural brain phenotypes ascertained by MRI. Finally, two-sample Mendelian randomisation analyses were performed to assess potentially causal effects of genetically-predicted cognition-associated protein levels on genetically-predicted neurological outcomes. For these analyses, genetic instrumental variables for protein levels were identified in the European, Latin, and Persian PURE participants, and associations with neurological outcomes were assessed using external (non-PURE) datasets. Created with BioRender.com.
Study findings
The PURE MIND and GS cohorts were similar in age, clinical characteristics, and sex distribution. A total of five proteins were associated with the digit symbol substitution task (DSST) performance in the PURE-MIND cohort.
The current study analyzed the association between the plasma levels of 1,160 proteins and cognitive function. Based on DSST measurements, carbonic anhydrase (CA14) and CUB domain-containing protein 1 (CDCP1) were associated with processing speed. More specifically, CA14 correlated with causal effects on hippocampal volume and increased risk of stroke, whereas CDCP1 was associated with intracranial aneurysms.
Proteins such as brevican (BCAN), neurocan (NCAN), and myelin-oligodendrocyte glycoprotein (MOG) were associated with DSST performance. These proteins have also been linked with structural brain phenotypes.
In this context, the cerebral white matter volume (WMV) plays a crucial role in mediating this effect. Previous studies have shown that WMH volume influences about 8% of the relationship between BCAN levels and DSST performance. Consistent with previous studies, the plasma levels of NCAN and BCAN were positively correlated with brain volume.
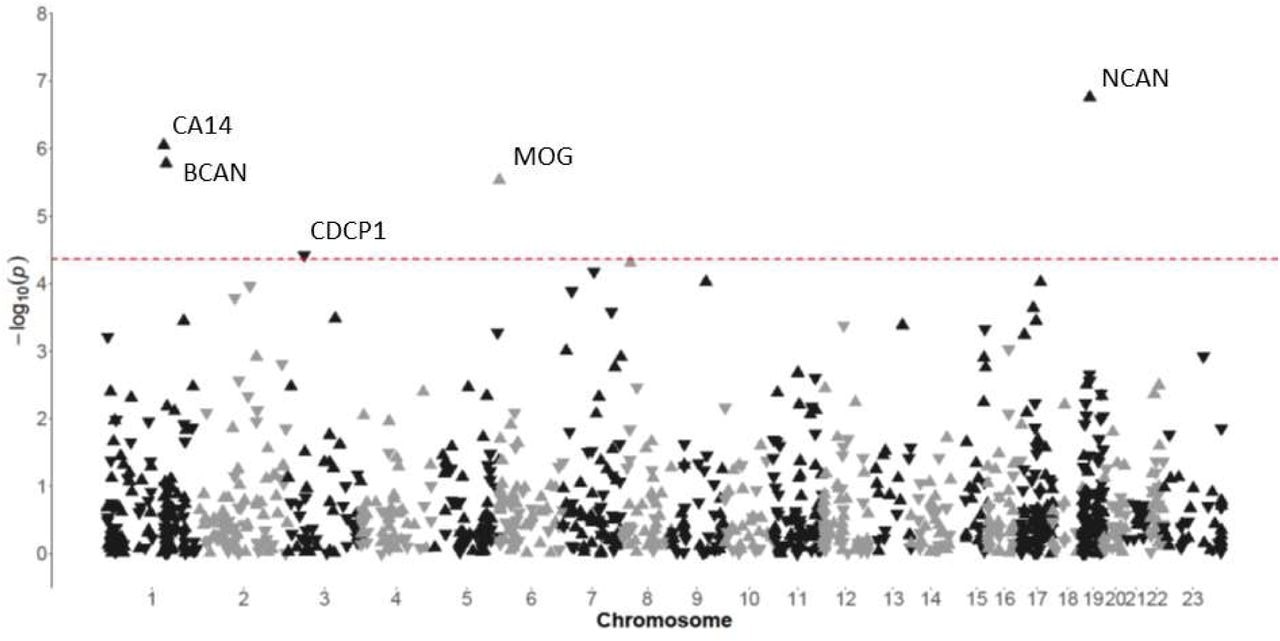 Manhattan plot indicating associations between the levels of plasma proteins and performance on the DSST in participants from the PURE-MIND cohort (N = 1198). Each protein is represented by a triangle with upwards-facing triangles indicating a positive association with DSST performance and downwards-facing triangles indicating a negative association with DSST performance. The position of each protein on the x-axis is determined by the genomic location of its corresponding gene and the position on the y-axis is determined by the –log10 p-value. The dashed horizontal line indicates the Bonferroni-corrected significance threshold (p = 4.31 x 10-5) required to maintain a 5% type I error rate.
Manhattan plot indicating associations between the levels of plasma proteins and performance on the DSST in participants from the PURE-MIND cohort (N = 1198). Each protein is represented by a triangle with upwards-facing triangles indicating a positive association with DSST performance and downwards-facing triangles indicating a negative association with DSST performance. The position of each protein on the x-axis is determined by the genomic location of its corresponding gene and the position on the y-axis is determined by the –log10 p-value. The dashed horizontal line indicates the Bonferroni-corrected significance threshold (p = 4.31 x 10-5) required to maintain a 5% type I error rate.
Based on the Mendelian randomization (MR) analyses, neuroprotective effects of carbonic anhydrase inhibition were observed in models of Huntington’s disease and amyloidosis, as well as ischemic and hemorrhagic stroke.
Enrichment analyses identified a notable association with DSST performance, which indicated higher levels of brain-expressed proteins. However, there was not a significant association between protein levels and the Montreal Cognitive Assessment (MoCA) performance. This finding implies the application of MoCA as a screening tool for mild cognitive impairment only.
CA14 is an isoform of the carbonic anhydrase family of zinc metalloprotease enzymes that catalyze the reversible hydration of carbon dioxide. This protein is expressed by neurons and is involved with the management of extracellular pH following synaptic transmission.
Of all carbonic anhydrase family members measured in this study, CA14 levels correlated with DSST performance. Nevertheless, more research is needed to determine the therapeutic potential of carbonic anhydrase for cognitive impairment, stroke, and Alzheimer’s disease.
Conclusions
The current study has some limitations including the consideration of proteins that represent a small subset of the circulating proteome. Replication analyses could not be performed for the entire cohort; therefore, the replication of CA14 and MOG were not assessed. Furthermore, protein levels were measured in the plasma, rather than the brain or cerebrospinal fluid (CSF).
Despite these limitations, the study findings highlight the importance of plasma for determining brain-related phenotypes. NCAN, BCAN, and their regulators were identified as molecules of interest in Alzheimer’s disease.
Future studies are needed to determine the underlying mechanisms that regulate the efflux of brain-expressed proteins into the bloodstream in non-clinical populations.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.