Sponsored Content by AbcamDec 15 2017
Active alpha-synuclein protein monomers and aggregates allow Lewy body pathology to be easily induced in neurons. They are suitable for both in vivo and in vitro experiments.
Lewy bodies – the intraneuronal aggregates of alpha-synuclein protein – are characteristics of Lewy body dementia, Parkinson’s disease and other medical disorders. They are found in the regions of the brain responsible for motor control and result in a progressive decline in mental ability.
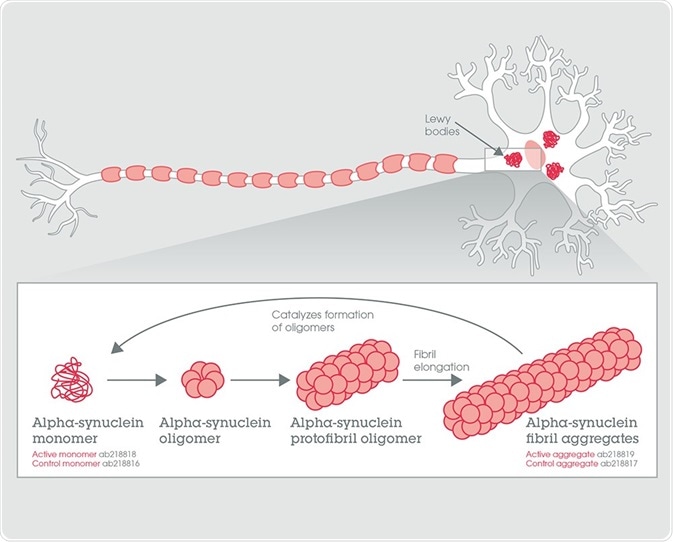
Figure 1. Alpha-synuclein fibril aggregate formation from the monomeric form.
The Advantages of Alpha-synuclein Proteins
- Distinguish active endogenous alpha-synuclein levels following the addition of aggregate or monomer proteins using thioflavin T (ThT) or a corresponding beta-sheet binding dye
- Induce Lewy body pathology in the experiment 1
- Explore both in vitro and in vivo applications (for example screen drug targets)
- Compare the results with alpha-synuclein aggregates or monomer controls
Assay Alpha-synuclein Proteins using Thioflavin T (ThT)
It is the active alpha-synuclein protein aggregate that seeds the development of novel alpha-synuclein aggregates from a pool of active alpha-synuclein protein monomers. The fluorescent dye ThT binds to beta sheet-rich structures, like those found in alpha-synuclein aggregates. Upon binding, the dye’s emission spectrum undergoes a red-shift and elevated fluorescence intensity.
Products
|
Product name
|
Ab ID
|
|
Recombinant human alpha-synuclein protein monomer (active)
|
ab218818
|
|
Recombinant human alpha-synuclein protein aggregate (active)
|
ab218819
|
|
Recombinant human alpha-synuclein protein monomer (control)
|
ab218816
|
|
Recombinant human alpha-synuclein protein aggregate (control)
|
ab218817
|
|
Thioflavin T (ThT)
|
ab120751
|
Alpha-synuclein Protein Assay Protocol
Procedure for assaying the binding of active alpha-synuclein aggregate and monomer to thioflavin T:
- 1 mM stock solution of ThT is prepared in dH2O (this should be prepared fresh and filtered through a 0.2 μm syringe filter).
- The ThT in PBS (pH 7.4) is diluted so that the final concentration of ThT in each well is 25 μM.
- The alpha-synuclein (aggregate and monomer) aliquots is thawed on ice just prior to use.
- Either 100 μM monomer or 10 nM aggregate (or both) is added to the appropriate wells.
- The plate is sealed and placed in a shaking incubator (600 rpm) at 37 °C.
- Using a fluorescence microplate reader (excitation 450 nm, emission 485 nm), the ThT fluorescence is measured from 1 hour up to 72 hours at 37 °C.
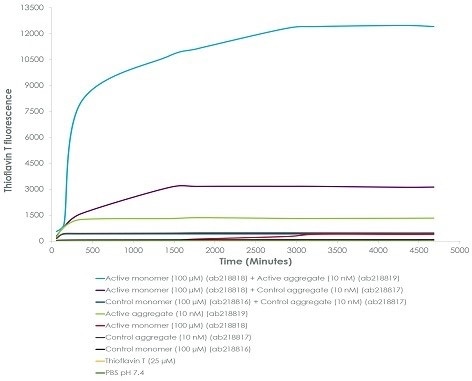
Emission curves of ThT show increased fluorescence (associated with the aggregation of alpha-synuclein protein) over time, when 100 µM of active alpha-synuclein monomer (ab218818) (light blue) is integrated with 10 nM of active alpha-synuclein aggregate (ab218819), as opposed to when 10 nM of control alpha-synuclein aggregate (purple line) is integrated with 100 µM of active alpha-synuclein monomer, or 10 nM of control alpha-synuclein aggregate (ab218817) (dark blue) is integrated with 100 µM of control alpha-synuclein monomer (ab218816). ThT ex = 450 nm, em = 485 nm.
Further Reading
- Luk, K. C. & Lee, V. M.-Y. Modeling Lewy pathology propagation in Parkinson’s disease. Parkinsonism Relat. Disord. 20, S85–S87 (2014).
- Vieira, B. D. M., Radford, R. A., Chung, R. S., Guillemin, G. J. & Pountney, D. L. Neuroinflammation in Multiple System Atrophy: Response to and Cause of α-Synuclein Aggregation. Front. Cell. Neurosci. 9, 437 (2015).
- Roberts, H. & Brown, D. Seeking a Mechanism for the Toxicity of Oligomeric α-Synuclein. Biomolecules 5, 282–305 (2015).
- Fernagut, P.-O. & Chesselet, M.-F. Alpha-synuclein and transgenic mouse models. Neurobiol. Dis. 17, 123–130 (2004).
- Ariesandi, W., Chang, C.-F., Chen, T.-E. & Chen, Y.-R. Temperature-Dependent Structural Changes of Parkinson’s Alpha-Synuclein Reveal the Role of Pre-Existing Oligomers in Alpha-Synuclein Fibrillization. PLoS One 8, e53487 (2013).
 About Abcam
About Abcam
Abcam is a global life sciences company providing highly validated antibodies and other binders and assays to the research and clinical communities to help advance the understanding of biology and causes of disease.
Abcam’s mission is to serve life scientists to help them achieve their mission faster by listening to their needs, continuously innovating and improving and by giving them the tools, data and experience they want. Abcam’s ambition is to become the most influential life science company for researchers worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.