With over 93 million infections confirmed and 2 million deaths reported by January 2021, the ongoing COVID-19 pandemic has caused deep devastation to the global community.
This severe acute respiratory illness is caused by a positive-sense single-stranded RNA virus, SARS-CoV-2, which is comprised of a genome of about 30K bp and interacts with the host cell through ACE2 binding via the viral spike protein.
So far, to tackle this disease, numerous vaccine candidates have been approved for emergency use, while a number of experimental medications are being explored for therapeutic treatment.
Additionally, accurate disease diagnostics, preventive measures and effective contact tracing are still considered essential for mitigating the spread of SARS-CoV-2 and worsening the pandemic.
One of the main strategies to manage the spread of SARS-CoV-2 is accurate disease diagnostics at the early stage of infection. As it stands, there are several lab tests available for detecting the SARS-CoV-2 virus. Among the accepted tests, RT-qPCR, and NGS-based tests (nucleic acid tests), are arguably the gold standard for good virus identification.
However, these tests are time-consuming and laborious and depend heavily on well-trained personnel, specialized instrumentation, as well as sophisticated laboratory facilities, resulting in their limited access and availability.
Conversely, the nucleic acid tests are not infallible since false-negative results are frequently observed due to reasons such as inappropriate sample handlings, storage conditions and sampling time. As a result, it is necessary to develop complementary assay formats to meet and expand the testing capacities.
Immunologic tests are workable solutions, and it has been reported that a combination of nucleic acid and immunologic tests considerably enhanced the sensitivity of pathogenic diagnosis for COVID-19 as early as the first week of contracting the disease.
These tests either look for pieces of virus proteins in the serum (antigen tests) or detect the antibodies against SARS-CoV-2 (serological tests). Immunologic tests can be conducted in standard clinical settings, and the results are available within a matter of hours, which significantly accelerates the diagnostic practices.
Serological tests
Serological tests (antibody tests) determine the presence of CoV-2 specific antibodies (mainly IgM and IgG classes) in a patient’s serum. Since IgM and IgG antibodies are the first-line responses in fighting viral infection in situ, they are the most practical biomarkers of SARS-CoV-2 serological diagnostics.
Figure 1 exhibits the timeline from SARS-CoV-2 infection to immunity by the presence of IgM and IgG antibodies in a patient’s serum.
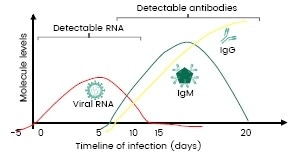
Figure 1. Timeline from SARS-CoV-2 infection to immunity generation (DOI: 10.1063/5.0021554). Image Credit: Sino Biological Inc.
Serological tests have extensive epidemiological and clinical significance. Employing these tests makes it possible to assess the duration and influence of immunity the virus causes, as well as evaluate the risk of re-infection, predict the potency of the vaccine and determine asymptomatic individuals.
Meanwhile, serological tests can also be used to enable the detection of convalescent plasma donors for the treatment of serious COVID-19 patients.
Up until September 2020, over 20 kinds of serological tests were approved by the NMPA in China, while more than 40 serological tests were approved by the FDA in US for emergency uses.
Such serological tests include rapid tests and non-rapid diagnostic tests. Rapid tests, predominantly lateral flow tests (LFA), have the capacity to identify antigen-specific IgG, and IgM or antigen-specific total antibodies in serum samples within just 30 minutes.
These tests are extremely beneficial due to low sample volume requirements, fast turn-around time, simplicity of use, as well as a low economic factor. When combined with additional advanced sensory technologies, the sensitivity of LAF methods has been considerably improved.
Non-rapid tests can offer clear quantification of the antibodies in the samples and they are generally more sensitive when compared to the LFA methods. The most frequently used non-rapid serological testing methods are enzyme-linked immunosorbent assay (ELISA) and chemiluminescence immunoassays (CLIA).
A general outline of the fundamental workflow of the aforementioned methods can be seen in Figure 2, while Table 1 outlines the pros and cons of each serological test format.
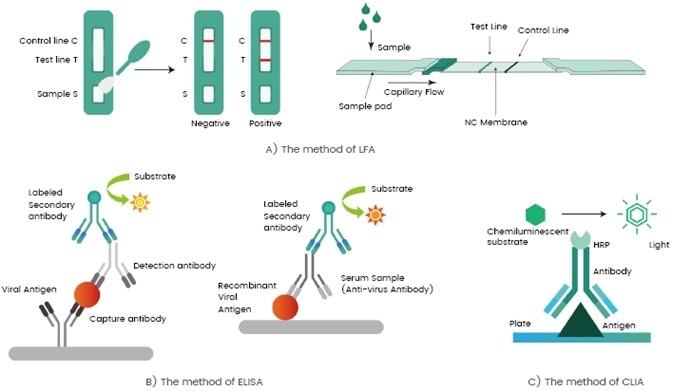
Figure 2. Serological test formats: rapid test, LFA (A); non-rapid tests ELISA (B) and CLIA (C). Image Credit: Sino Biological Inc.
Table 1. Comparison of serological testing methods. Source: Sino Biological Inc.
| Method |
LFA |
ELISA |
CLIA |
| Read-out |
Semi-quantitative |
Quantitative |
Quantitative |
| High throughput |
No |
Yes |
Yes |
| Ease of use |
Easy |
Moderate |
Hard |
| Accuracy |
low |
Moderate |
high |
| Cost |
low |
Moderate |
high |
Sensitivity and specificity are the point of interest of any serological tests, and they are greatly influenced by the core material and its quality, i.e., the recombinant proteins used in the assay developments.
Recombinant proteins that are very similar to their naturally occurring counterparts will boost the accuracy of the test significantly. For SARS-CoV-2, the most copious amounts of viral proteins in circulation are spike (S) and nucleocapsid protein (NP).
These proteins are also the primary triggers of the body’s antibody responses, and they can both act as crucial ingredients for serological test developments.
Utilizing recombinant protein expression technology, Sino Biological has produced biologically active and stable Spike (40591-V08H, 40592-V05H) and NP proteins (40588-V08B, 40588-V07E) of SARS-CoV-2 (Figure 3) with great success.
Meanwhile, a collection of the S and NP proteins from various other coronavirus strains are also available as core components in a number of commercial Serological diagnostic kits.
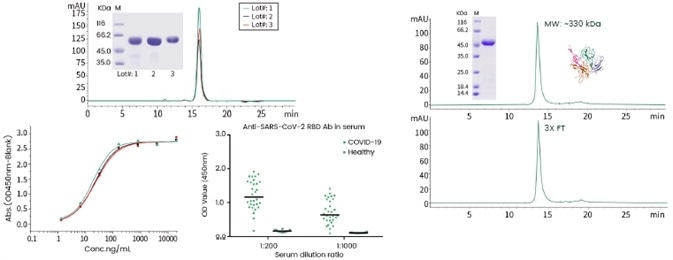
Figure 3. Examples of high-quality recombinant SARS-CoV-2 sand NP. Left) The RBD domain of the Spike protein was expressed in HEK293 via transient transfection. The high purity proteins with confirmed ACE2 binding activity exerted minimum batch-to-batch variance and applicable to detect anti-CoV-2 antibodies from patient serum samples. Right) The full-length NP was expressed in E.coli (40588-V07E). The protein was stable after 3X freeze-thaw cycles and presented as an oligomer. Image Credit: Sino Biological Inc.
Antigen tests
When infection occurs, the presence of viral antigens (pieces of virus proteins) can be seen before the host’s antibody responses kick in. Therefore, antigen-based immunoassays would be useful for the early diagnosis of COVID-19. NP is the primary target for antigen test developments for SARS-CoV-2.
The FDA authorized 13 antigen diagnostic tests for emergency uses. Like serological tests, specificity and sensitivity are also the key factors in antigen tests and they are largely determined by the antibodies used in test developments.
In terms of assay formats, certain assay arrangements employed in serologic tests are also relevant for antigen tests, including immunofluorescence assays (IFAs), LFA and CLIA.
Utilizing sophisticated antibody generation platforms, Sino Biological has created a large panel of antibodies against the NP of SARS-CoV-2. Upon comprehensive screening efforts, Sino Biological has identified a number of antibody pairs with high specificities and sensitivities towards NP.
These antibody pairs are employed as material for ELISA-based antigen tests (Figure 4, left). Meanwhile, some antibodies with phantom-molar sensitivities have also appeared during the planning process, and they have been shown to be compatible in ultra-sensitive detection platforms, including Simo, Figure 4 right.
Table 2. Source: Sino Biological Inc.
| |
Capture Ab (Cat#) |
Detection Ab (Cat#) |
 |
| Pair1 |
40143-MM05 |
40588-R001 |
| Pair 2 |
40588-MM123 |
40588-R001 |
| Pair 3 |
40143-MM05 |
40143-R001 |
| Pair 4 |
40143-MM08 |
40143-R004 |
| Pair 5 |
40143-R004 |
40143-R040 |
Table 3. Source: Sino Biological Inc.
| Antigen |
Capture Ab (Cat#) |
Detection Ab (Cat#) |
Standard (cat#) |
LOD |
| N |
40143-MM05 |
40143-R001 |
40588-V08B |
15.7 fg/ml |
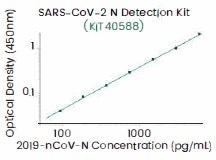
Figure 4. Recommended antibody pairs for ELISA-based quantitative antigen test kits (left) and ultra-sensitive single molecular array (SIMOA, right). Image Credit: Sino Biological Inc.
Challenge and solutions
The impact of SARS-CoV-2 variants
RNA viruses are renowned for their rapid mutation rates. The evolving nature of the viral proteome produces a significant impact on the accuracy of immunological assays. It is critical to evaluate the feasibility of the present assay materials in adaptation to the virus mutagenesis.
Several SARS-CoV-2 variants with multiple mutations have now been recorded. One strain, categorized in the or 20B/501V.Vl lineage has raised concerns since evidence indicates that it showed the potential to evade vaccine or immunological diagnosis.
To confirm the effectiveness of the current immunological diagnostic reagents, specifically antibodies used for antigen tests, scientists at Sino Biological have produced the NP protein of the B.l.1.7 UK strain and tested the performance of the current anti-NP antibodies (Figure 6).
Results demonstrated that current antibodies performed similarly against the main mutations of B.l.1.7 of NP.
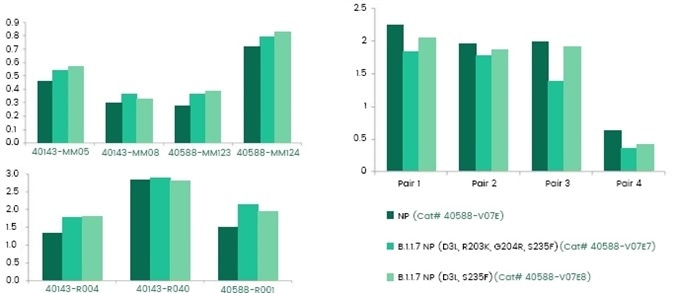
Figure 6. Activities of anti-NP antibody (left) and antibody pairs (right) against NP from UK variant. Image Credit: Sino Biological Inc.
Antibody cross-reactivity
The NP of SARS-CoV-2 demonstrated modest sequence similarities (60~65%) against CoV strains that induce strains of seasonal flu while it has ~75% and ~97% sequence similarity against MERS and SARS CoV, respectively.
Thus, it is difficult when trying to develop a very specific SARS-CoV-2 immunological test with low cross-reactivity against other CoV strains.
Sino Biological has developed NP for seven CoV strains and used these proteins as screening agents successfully. The company has distinguished a number of SARS-CoV-2 NP individual monoclonal antibodies with negligible cross-reactivity, as shown in Figure 7.
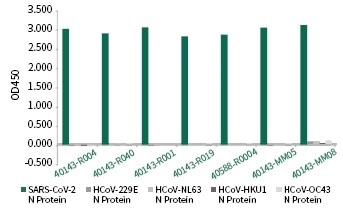
Figure 7. Cross-reactivity of SARS-CoV-2 specific Anti-N antibodies. Image Credit: Sino Biological Inc.
References
- https://www.acpjournals.org/doi/full/10.7326/M20-1495
- https://doi.org/10.3389/fimmu.2020.610688
- https://doi.org/10.1093/cid/ciaa344
- https://doi.org/10.1101/2020.03.24.006544
- https://aip.scitation.org/doi/10.1063/5.0021554
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.