Cancer is the second leading cause of death worldwide and is thus considered a truly global challenge. In 2022 alone, there will be 1.9 million new cancer cases and 609,360 deaths from cancer in the United States, according to estimates from the American Cancer Society.
Immunotherapy harnesses the immune system to fight cancer cells, unlike traditional cancer treatments such as surgery, chemotherapy, and radiotherapy, and has emerged as the forefront treatment (standard treatment) for many solid tumors, including lung cancer, melanoma, bladder cancer, and head and neck cancer.1
Researchers in industry and academia, inspired by the great success of PD-1/PD-L1 inhibitors, are collaborating on the identification of other novel target molecules like CD47, T-cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT), and signal regulatory protein α (SIRPα) in order to maximize the efficacy of immunotherapy.
Thanks to its central role in limiting antitumor responses, TIGIT is an attractive target for cancer immunotherapy.2
A member of the poliovirus receptor (PVR)/nectin family, TIGIT has several ligands which are also shared by other receptors like CD226 (DNAM-1), CD112R, and CD96 (TACTILE). These ligands include CD155 (PVR), CD112, and CD113: of which TIGIT has a higher affinity for CD155 than for CD112 and CD113.
Expressed on activated T cells and NK cells, TIGIT binds to CD155 on APCs and in various solid and hematologic tumors (Figure 1). A series of anti-TIGIT agents are currently undergoing clinical development for cancer therapy, as displayed in Table 1.
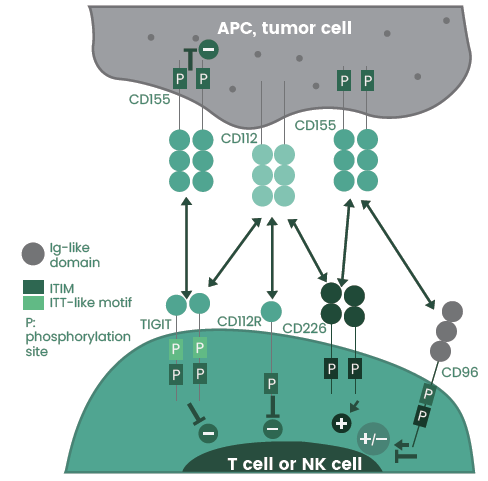
Figure 1. The TIGIT/CD226/CD96/CD112R axis. Image Credit: Sino Biological Inc.
Table 1. Anti-TIGIT agents under clinical development. Source: Sino Biological Inc.
| Name |
Type |
Company |
Status |
| Tiragolumab (MTIG7192A) |
Fully human IgG1 |
Roche |
Phase III |
| Ociperlimab (BGB-A1217) |
Humanized IgG1 |
BeiGene |
Phase III |
| Vibostolimab (MK-7684) |
Humanized IgG1 |
Merck |
Phase II |
| Domvanalimab (AB154) |
Humanized IgG1 |
Arcus Biosciences |
Phase III |
| BMS-986207 |
Fully human IgG1 |
Bristol-Myers Squibb |
Phase II |
| EOS-448 |
Fully human IgG1 |
iTeos Therapeutics |
Phase II |
| ASP-8374 |
Fully human IgG4 |
Astellas Pharma |
Phase I |
| COM-902 |
Fully human IgG4 |
Compugen |
Phase I |
| Etigilimab |
Fully human IgG1 |
Mereo Biopharma |
Phase I |
| Tamgiblimab (IBI-939) |
NA |
Innovent Biologics |
Phase I |
NA: details not available.
The most advanced stage of development (phase III) features tiragolumab, ociperlimab, and domvanalimab. A fully human IgG1 monoclonal antibody, tiragolumab is designed to bind TIGIT and block its interaction with CD155.
ITiragolumab plus atezolizumab displayed notable clinical benefits regarding progression-free survival and overall survival as opposed to atezolizumab alone3 in a phase II study of first-line treatment for PD-1 high non-small-cell lung cancer (NSCLC).
In January 2021, the FDA granted the combination Breakthrough Therapy Designation. Roche has continued to examine tiragolumab in the treatment of NSCLC despite its phase III failure in May 2022.
A humanized IgG1 antibody, ociperlimab with high affinity and specificity, binds to TIGIT. The comparison is between ociperlimab plus tislelizumab, versus pembrolizumab alone, in previously untreated patients with advanced NSCLC in a phase III trial (NCT04746924).4
An Fc-silent humanized IgG1 antibody, Domvanalimab poses less of a risk of depleting intratumoral CD8+ effector T cells. Arcus Biosciences has planned various phase III studies to further evaluate combination regimens across different cancer types, as domvanalimab displayed promising efficacy.
Sino Biological provides high-quality TIGIT proteins from various species such as rats, humans, and cynomolgus monkeys (Figure 2) to support the discovery and development of TIGIT-targeting therapeutic agents). For researchers who want to conduct in-depth studies of the TIGIT axis, CD96, CD155, and CD226 proteins are also available.
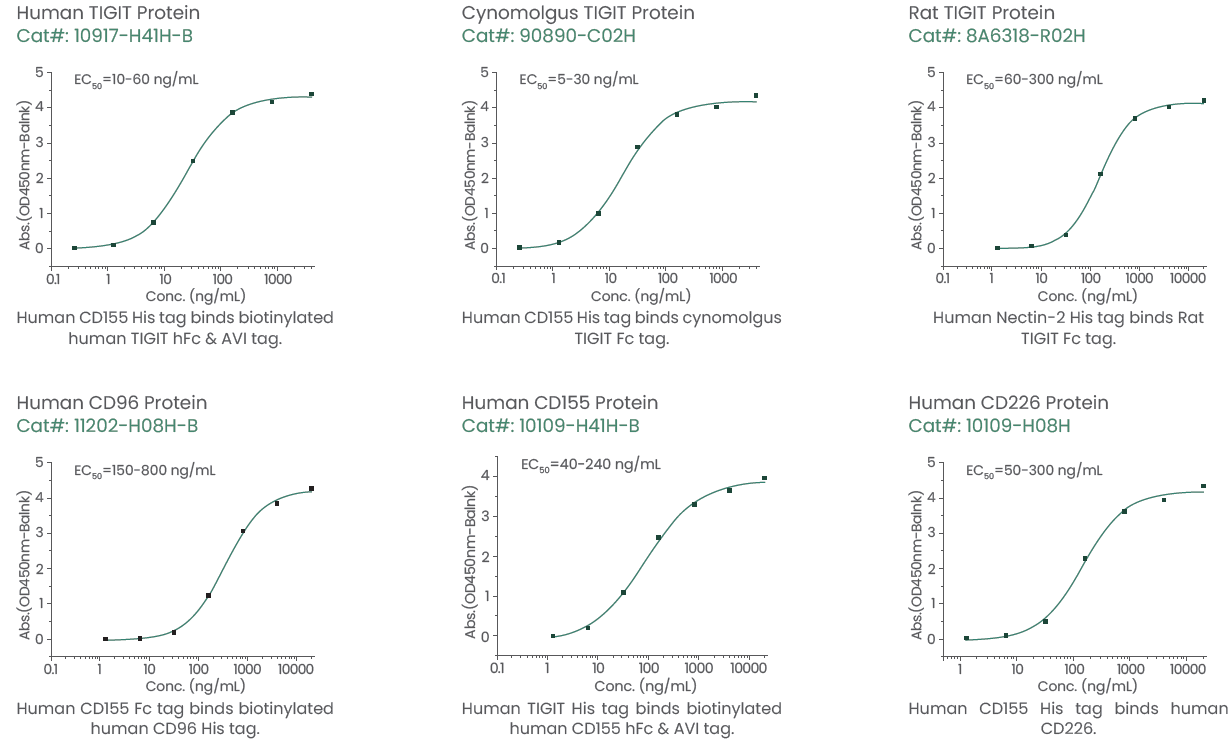
Figure 2. Featured TIGIT, CD96, CD155, and CD226 proteins. Image Credit: Sino Biological Inc.
CD47–SIRPα
Whilst SIRPα is especially abundant in dendritic cells, macrophages, and neutrophils, CD47 is broadly expressed in both hematological malignancies and solid tumors. CD47 triggers a potent intracellular “don’t eat me” signal to immune effector cells when tumor cells bind to SIRPα, which thereby evades immune surveillance and inhibits phagocytic cell activation.
Blocking the CD47–SIRPα interaction is, therefore, an emergent and highly promising therapeutic strategy which may be used to promote antitumor immunity (Figure 3).
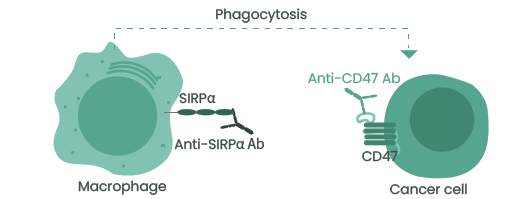
Figure 3. Anti-CD47 or SIRPα antibodies induce the phagocytosis of tumor cells by macrophages. Image Credit: Sino Biological Inc.
CD47–SIRPα
A range of CD47/SIRPα inhibitors, including anti-CD47 or SIRPα monoclonal antibodies, SIRPα–Fc fusion proteins, and bispecific antibodies (Table 2), have been developed and evaluated in clinical trials. However, there has not as yet been a therapeutic agent brought to market that blocks the CD47–SIRPα signaling pathway.
Table 2. Select inhibitors of the CD47–SIRPα axis under clinical development. Source: Sino Biological Inc.
| Name |
Type |
Company |
Status |
Indication |
| Magrolimab (Hu5F9-G4) |
CD47 mAb |
Forty Seven |
Phase III |
MDS, AML |
| Letaplimab (IBI188) |
CD47 mAb |
Innovent Biologics |
Phase III |
MDS, AML |
| Ligufalimab (AK117) |
CD47 mAb |
Akesobio Biopharma |
Phase II |
MDS, AML |
| Lemzoparlimab (TJC4) |
CD47 mAb |
I-Mab BIOPARMA |
Phase I/II |
Solid tumors, AML, MDS |
| AO-176 |
CD47 mAb |
Arch Oncology |
Phase II |
Solid tumors, MM |
| IBI322 |
CD47/PD-L1 bsAb |
Innovent Biologics |
Phase I |
Advanced malignancies |
| HX009 |
CD47/PD-L1 bsAb |
Waterstone Hanxbio |
Phase II |
Solid tumors |
| Evorpacept (ALX148) |
SIRPα fusion protein |
ALX Oncology |
Phase II/III |
Solid tumors, MDS |
| TTI-621 |
SIRPα fusion protein |
Trillium Therapeutics |
Phase I/II |
Solid tumors |
| GS-0189 |
SIRPα mAb |
Forty Seven |
Phase I |
NHL |
| CC-95251 |
SIRPα mAb |
Celgene |
Phase I |
Solid tumors, hematological
malignancies |
MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma
A humanized anti-CD47 IgG4 antibody, Magrolimab, is currently in phase III development, which could be the first agent of its kind to gain approval.
CD47-targeting agents induce the hemagglutination and phagocytosis of RBCs in vitro because CD47 is expressed on red blood cells (RBCs), which indicates potential toxicity. Patients initially received a low priming mAb dose (1 mg/kg) to occupy the RBC sink and induce compensatory erythropoiesis in clinical trials of magrolimab, thereby facilitating tolerance of subsequent higher maintenance doses (30 mg/kg).5
Magrolimab plus rituximab produced overall and complete response rates of 49 and 21%, respectively, in a phase Ib/II study evaluating patients with non-Hodgkin’s lymphoma (NHL).6
Another CD47 mAb in phase III development, Letaplimab, is a specific blocker of the CD47–SIRPα axis. Letaplimab displayed a favorable toxicity profile without dose-limiting toxicities in a phase I study of patients with advanced solid tumors and lymphomas. Most of the adverse events were measured at grade 1–2 in severity, with an overall anemia rate of 15%, and only one patient experiencing grade 3 anemia.7
IBI322, in addition to letaplimab, is also being developed by Innovent Biologics. IBI322 is an anti-human CD47/PD-L1 bispecific antibody that can bind CD47+PD-L1+ tumor cells selectively, which therefore reduces the potential side effects that the CD47 blockade causes in healthy cells.8
In order to evaluate its safety, tolerability, and pharmacokinetics in patients with advanced solid tumors, IBI322 is currently under phase I development.
By fusing the CD47 binding domain of SIRPα to the Fc region of human IgG1, SIRPα–Fc fusion proteins targeting CD47 are engineered, including ALX148 and TTI-621. ALX148 demonstrated, in preclinical studies, a potent antitumor efficacy in murine models using human tumor xenografts.9
In a similar manner, TTI-621 effectively suppressed the growth of B lymphoma xenografts and aggressive acute myeloid leukemia.10 GS-0189 and CC-95251 are several of the anti-SIRPα monoclonal antibodies that are in the early stage of clinical development.
The IgV domain of SIRPα is highly polymorphic, and within humans, there are ten allelic variants. The most prevalent variants have been reported as SIRPα V1, SIRPα V2, and SIRPα V8, which are thought to account for more than 90% of all forms in the human population.11-12
SIRPγ is closely related to SIRPα but has a weaker affinity than SIRPα when binding to CD47 and is involved in T-cell responses.13
In the development of therapeutic antibodies, it is essential to assess the binding to various SIRPα variants. High-quality SIRPα V2, SIRPα V8, and SIRPγ proteins have been produced by Sino Biological, and Figure 4 presents the product data. Mouse, rat, and human CD47 proteins are also available.
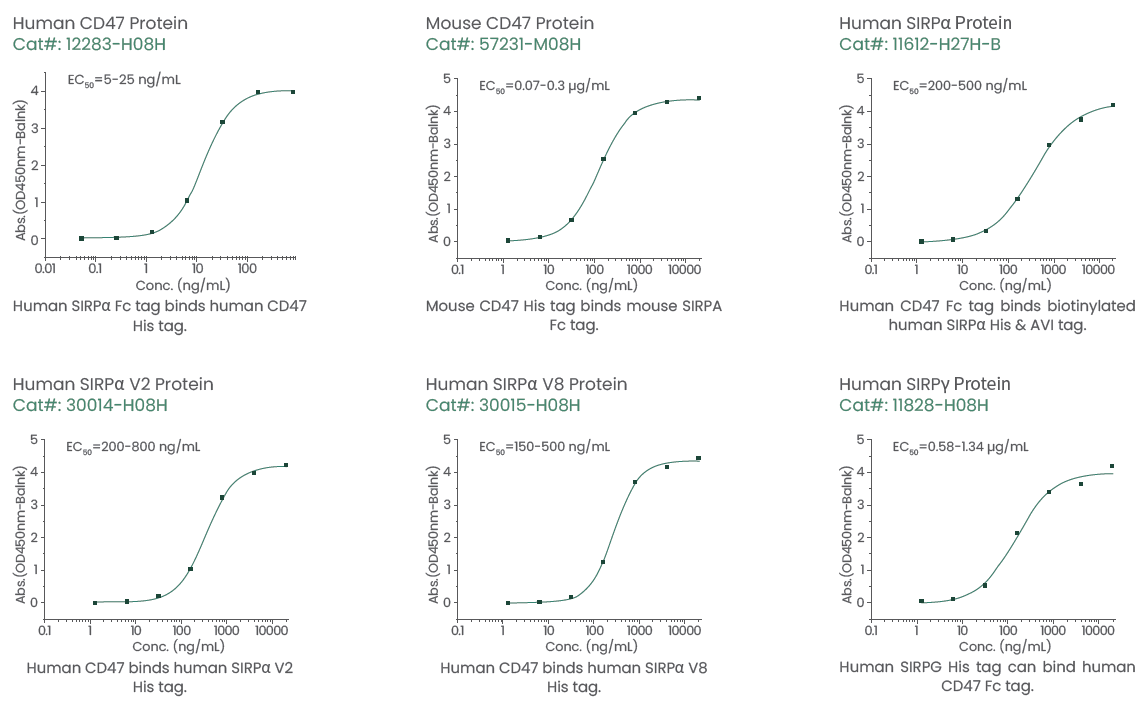
Figure 4. Highly active recombinant CD47, SIRPα, and SIRPγ proteins. Image Credit: Sino Biological Inc.
Conclusion
It must be said that cancer immunotherapy is a rapidly developing field in which there have been a number of recent significant advancements. Favorable antitumor efficacy and good safety profiles have been detected for both preclinical- and clinical-stage drugs14-15 that act upon other emerging targets, including TIM-3, VISTA, LAG-3, and CD27 (Table 3), in addition to the aforementioned targets.
A range of truly effective new treatment strategies is likely to be available to patients with cancer in the future, thanks to this continued exploration of novel therapeutic targets or combination strategies.
Table 3. Summary of additional emerging immunotherapy targets. Source: Sino Biological Inc.
| |
| TIM-3 |
VISTA |
LAG-3 |
B7-H3 |
ICOS |
ICOS-L |
BTLA |
| CD27 |
CD70 |
HHLA2 |
B7-H4 |
CD137 |
CD137L |
IDO |
| CD94 |
NKG2A |
OX40 |
OX40L |
CD40 |
CD40L |
GITR |
References
- doi: 10.3747/co.25.3840
- doi: 10.1111/cei.13407
- doi: 10.1007/s11912-022-01281-5
- doi: 10.1200/JCO.2021.39.15_suppl.TPS9128
- doi: 10.1200/JCO.2022.40.16_suppl.7054
- doi: 10.1186/s13045-021-01197-w
- doi: 10.3390/cancers13246229
- doi: 10.1007/s00432-020-03404-6
- doi: 10.1371/journal.pone.0201832
- doi: 10.1158/1078-0432.CCR-16-1700
- doi: 10.1172/jci.insight.134728
- doi: 10.1186/s40364-022-00373-5
- doi: 10.1186/s40425-019-0772-0
- doi: 10.1016/j.semcancer.2017.10.001
- doi: 10.2217/imt.15.78
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.