Recent progress in the development of therapeutic monoclonal antibodies (mAbs) has resulted in effective treatment across a range of different types of cancer and inflammatory disorders.
It is well documented that monoclonal antibodies shape a crucial defense barricade against numerous threats, from environmental pathogens, foreign objects to in situ oncogenic entities.
A monoclonal antibody distinguishes its antigen via extremely specific intermolecular interactions and thereafter recruits serum immune cells to eradicate targets via antibody-associated effector functions, specifically antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
Comprehensive studies have recently been geared towards ADCC and how it may be improved for therapeutic applications. For instance, a mAb unleashes the power of effector cells to eliminate its target upon the interaction between the mAb Fc domain and the FcvRs on the effector cell surface.
The ADCC of a mAb can be modified with changes to the Fc region and one of such approaches is via Fc glycan chain engineering. Said engineering is a novel and inventive way to both enhance and target mAb therapeutic action.
In response to these studies, ADCC is now one of the main modes of action for many current therapeutic antibodies. It is the principal mechanism that determines and drives the efficacy of an antibody and is, therefore, a key factor for its therapeutic potential.
As previously mentioned, the structure and composition of the mAb glycan content can sway its ADCC activity. Specifically, an absence of core fucose residues on the Fc N-glycan moiety has demonstrated the potential to increase IgG1 Fc binding affinity to CD16 (Fc gamma receptor IIIa).
This receptor presents on immune effector cells such as natural killer cells, and said binding by IgG1 results in increased ADCC activity. The removal of the fucose residues was first achieved via a blend of chemical and enzymatic treatment of the mAb, a labor-intensive process with high operational costs.
Recently, the finding of the gene responsible for the addition of the fucose residues, fucosyltransferase 8 (FUT8), along with progress in gene manipulation techniques, make it possible to generate antibody-expressing cell lines with anFUT8 gene knockout for the manufacture of afucosylated mAbs (Figure 1).
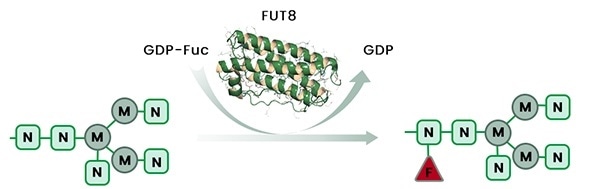
Figure 1. The removal of glycan core fucose residues is achieved by knockout of the host cell fucosyltransferase-8 (FUT8) gene. Image Credit: Sino Biological Inc.
Establishment of FucoFree system
Given the significance and impact of glycan engineering enhancement of mAbs for therapeutic use, researchers at Sino Biological have utilized such gene editing methods to determine a proprietary “FucoFree” eukaryotic cell culture system for the custom expression of afucosylated mAbs.
These FucoFree cell lines have been tested rigorously both at the genetic level to confirm the exhaustive knockout of the FUT8 gene and at the protein level to verify the absence of fucose residues in the antibody glycan chain.
Host cells in the FucoFree system include a FUT8 knockout (FUT-/-) CHO cell line and a FUT-/- HEK293 cell line, engineered individually to meet the demands of various projects and scales of production (Figure 2).
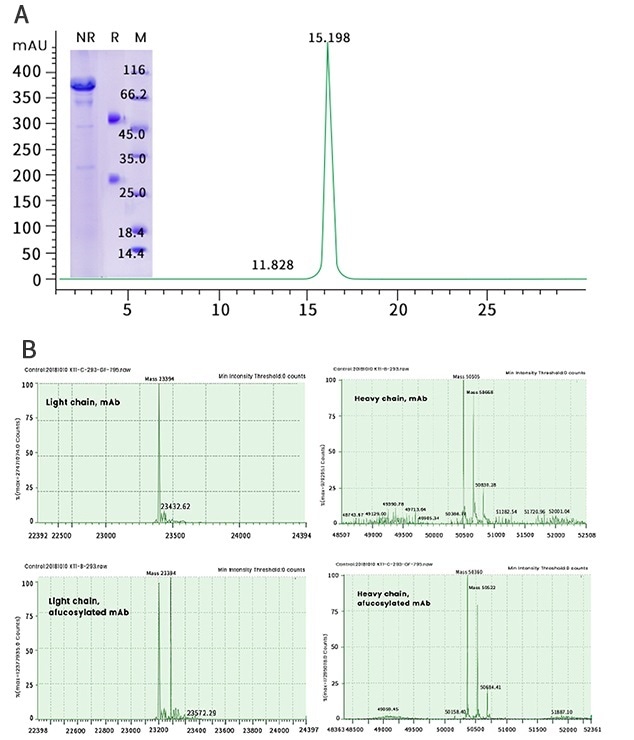
Figure 2. High-quality Antibodies Produced in “FucoFree” Cell Lines. A) Recombinant antibody demonstrating >95% purity and >98% monomer distribution. (B) LC-MS analysis of a mAb expressed in a conventional (two top panels in B) and the corresponding“FucoFree” (two bottom panels in B) cell line. A difference in the heavy chain molecular weight of 145 Da was observed, corresponding well with the MW of a fucose residue. Image Credit: Sino Biological Inc.
The FucoFree system is a first-class antibody/recombinant protein expression platform in a number of ways, including:
- Afucosylated antibody
- Exceptional development and licensing potential
- Full-spectrum antibody characterization
- High-yield and high-throughput antibody expression
- Long-standing gene knockout technology
- Multi-scale antibody production
Through the application of the FucoFree system, Sino Biological can provide a comprehensive yet dynamic service package designated to address:
- ADCC-enhanced mAb candidate screening
- mAb ADCC assessment
- mAb expression using custom sequences
- mAb glycan profiling
- Stable expression cell line establishment
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.